Cancer Res Treat.
2005 Oct;37(5):268-272.
Results of Curative Radiation Therapy with or without Chemotherapy for Stage III Unresectable Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Chonnam National University Medical School, Gwangju, Korea. ahnsja@chonnam.ac.kr
- 2Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Internal Medicine, Seonam University Medical School, Gwangju, Korea.
Abstract
- PURPOSE
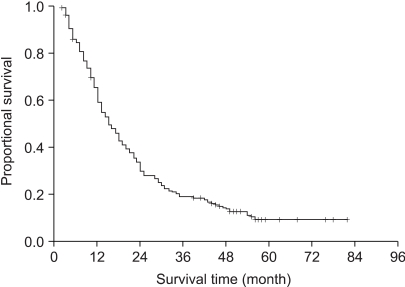
We retrospectively analyzed the patients who received curative radiotherapy for unresectable stage III NSCLC to investigate the impact of chemotherapy. MATERIALS AND METHODS: From 1998 to 2001, the records of 224 patients who completed curative radiotherapy for NSCLC were reviewed. There were 210 males and 14 females, and their median age was 64 years (range 38 ~83). 54 patients had stage IIIA disease and 170 patients had stage IIIB disease. Conventional radiotherapy was given and the radiation dose ranged from 50~70 Gy with a median of 60 Gy, and chemotherapy was combined for 116 patients (52%). RESULTS: The median survival, the 2-year, and 5-year actuarial survival rates of all 224 patients were 15 months, 30%, and 7%, respectively. The median survival of the patients with stage IIIA and IIIB disease were 21 months and 13 months, respectively (p=0.14). The median survival of patients who received chemoradiation was 18 months compared to 14 months for the patients who received RT alone (p=0.02). Among the chemoradiation group of patients, the median survival time of the patients who received 1 to 3 cycles of chemotherapy was 16 months and that for the patients who received more than 3 cycles was 22 months (p=0.07). We evaluated the effects of the timing of chemoradiation in 57 patients who received more than 3 cycles of chemotherapy. The median survival of the patients with the concurrent sequence was 25 months and that for the patients with the sequential chemotherapy was 19 months (p=0.81). CONCLUSIONS: For advanced stage III non-small cell lung cancer patients who completed the curative radiotherapy, the addition of chemotherapy improved the survival compared to the patients who received radiotherapy alone.
MeSH Terms
Figure
Reference
-
1. Choy H, Akerley W, Safran H, Graziano S, Chung C, Williams T, et al. Multiinstitutional phase II trial of paclitaxel, carboplatin, a concurrent radiation therapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 1998; 16:3316–3322. PMID: 9779707.2. Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III NSCLC: Seven year follow-up of CALGB 8433 trial. J Natl Cancer Inst. 1996; 88:1210–1215. PMID: 8780630.3. Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in non-resectable non-small cell lung cancer: First analysis of a randomized trial of 353 patients. J Natl Cancer Inst. 1991; 83:417–423. PMID: 1847977.4. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized clinical trials. Br Med J. 1995; 311:899–909. PMID: 7580546.5. Sause W, Kolesar P, Taylor S IV, Johnson D, Livingston R, Komaki R, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000; 117:358–364. PMID: 10669675.6. Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999; 17:2692–2699. PMID: 10561343.
Article7. Curran W, Scott C, Langer R, Komaki R, Lee JS, Movsas B, et al. Phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III non-small cell lung cancer (NSCLC): Report of Radiation Therapy Oncology Group (RTOG) 9410. Lung Cancer. 2000; 29:93. (abstr 303).8. Gaspar LE. Optimizing chemoradiation therapy approaches to unresectable stage III non-small cell lung cancer. Curr Opin Oncol. 2001; 13:110–115. PMID: 11224708.
Article9. Komaki R, Seiferheld W, Ettinger D, Lee JS, Movsas B, Sause W. Randomized phase II chemotherapy and radiotherapy trial for patients with locally advanced inoperable non-small-cell lung cancer: long-term follow-up of RTOG 92-04. Int J Radiat Oncol Biol Phys. 2002; 53:548–557. PMID: 12062596.
Article10. Arriagada R. Current strategies for radiation therapy in non-small cell lung cancer. Chest. 1997; 112(4 Suppl):209S–213S. PMID: 9337291.
Article11. Kaplan B, Altynbas M, Eroglu C, Karahacioglu E, Er O, Ozkan M, et al. Preliminary results of a phase II study of weekly paclitaxel (PTX) and carboplatin (CBDCA) administered concurrently with thoracic radiation therapy (TRT) followed by consolidation chemotherapy with PTX/CBDCA for stage III unresectable non-small-cell lung cancer (NSCLC). Am J Clin Oncol. 2004; 27(6):603–610. PMID: 15577439.
Article12. Gandara DR, Chansky K, Albain KS, Leigh BR, Gaspar LE, Lara PN, et al. Consolidation docetaxel after concurrent chemoradiotherpy in stage IIIB non-small- cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003; 21:2004–2010. PMID: 12743155.13. Bradley J, Graham MV, Winter K, Purdy JA, Komaki R, Roa WH, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005; 61:318–328. PMID: 15667949.14. Rengan R, Rosenzweig KE, Venkatraman E, Koutcher LA, Fox JL, Nayak R, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004; 60:741–747. PMID: 15465190.
Article15. De Candis D, Stani SC, Bidoli P, Bedini VA, Potepan P, Navarria P, et al. Induction chemotherapy with carboplatin/paclitaxel followed by surgery or standard radiotherapy and concurrent daily low-dose cisplatin for locally advanced non-small cell lung cancer (NSCLC). Am J Clin Oncol. 2003; 26:265–269. PMID: 12796598.
Article16. Trodella L, Granone P, Valente S, Margaritora S, Macis G, Cesario A, et al. Neoadjuvant concurrent radiochemotherapy in locally advanced (IIIA-IIIB) non-small-cell lung cancer: long-term results according to downstaging. Ann Oncol. 2004; 15:389–398. PMID: 14998840.
Article17. Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, Weick JK, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stage IIIA(N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995; 13:1880–1892. PMID: 7636530.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phase II Study of Concurrent Chemotherapy with Etoposide and Cisplatin (EP) and Radiation Therapy for Unresectable Stage III Non-small Cell Lung Cancer
- Role of Radiation Therapy for Non-small Cell Lung Cancer: Focused on Stereotactic Ablative Radiation Therapy in Stage I
- Management of Locally Advanced Non-small Cell Lung Cancer
- Chemo-radiation Therapy for Locally Advanced Non-Small Cell Lung Cancer
- The Results of Radiation Therapy in Stage III Non-Small Cell Lung Cancer