Ann Dermatol.
2016 Jun;28(3):304-313. 10.5021/ad.2016.28.3.304.
Protective Effect of Topical Vitamin D3 against Photocarcinogenesis in a Murine Model
- Affiliations
-
- 1Department of Dermatology, Dankook University College of Medicine, Cheonan, Korea. zamoo97@naver.com
- 2Department of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2164637
- DOI: http://doi.org/10.5021/ad.2016.28.3.304
Abstract
- BACKGROUND
Although the incidence of non-melanoma skin cancer is increasing, there are no effective practical preventive measures other than avoiding sun exposure.
OBJECTIVE
To elucidate the protective effect of topical application of biologically active vitamin D3 (calcitriol) on skin cancer development caused by exposure to ultraviolet (UV).
METHODS
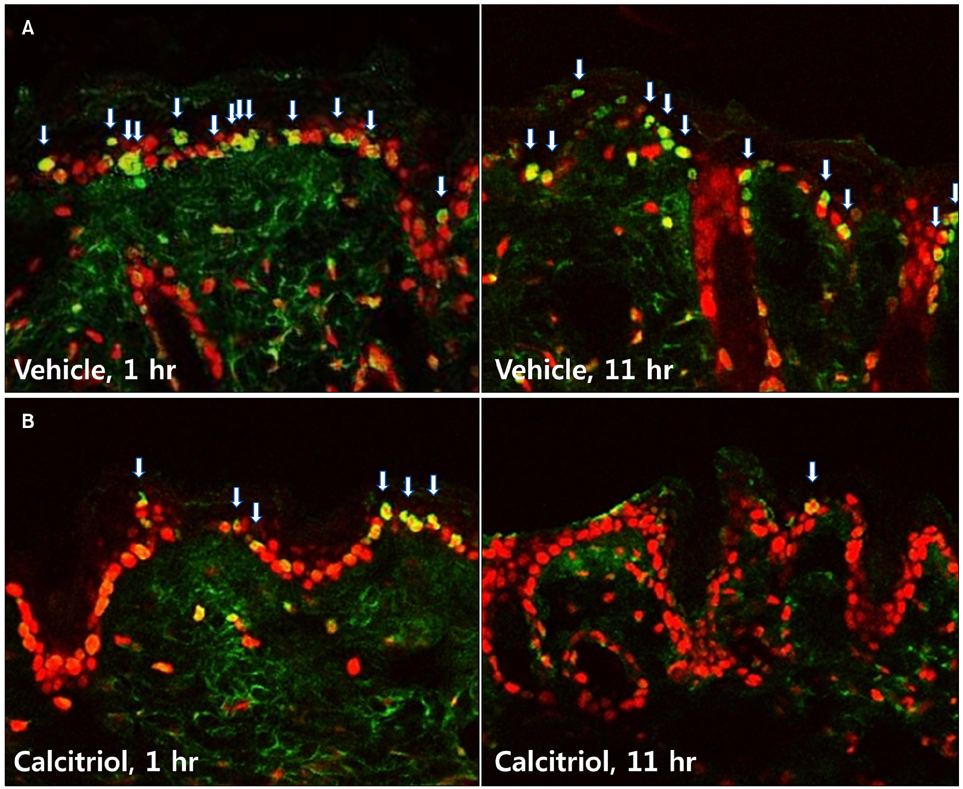
Groups of hairless mice were topically treated with either calcitriol or vehicle immediately after exposure to UVB and UVA three times weekly for the initial 20 weeks, and without UV exposure in the following 6 weeks. Tumor number was counted and biopsies were done for histopathologic analysis. The changes of cyclobutane pyrimidine dimer (CPD) were evaluated 1 hour and 11 hours after short term of UV exposure and application of calcitriol. For safety evaluation, blood test and body weights were evaluated at 23rd and 25th week.
RESULTS
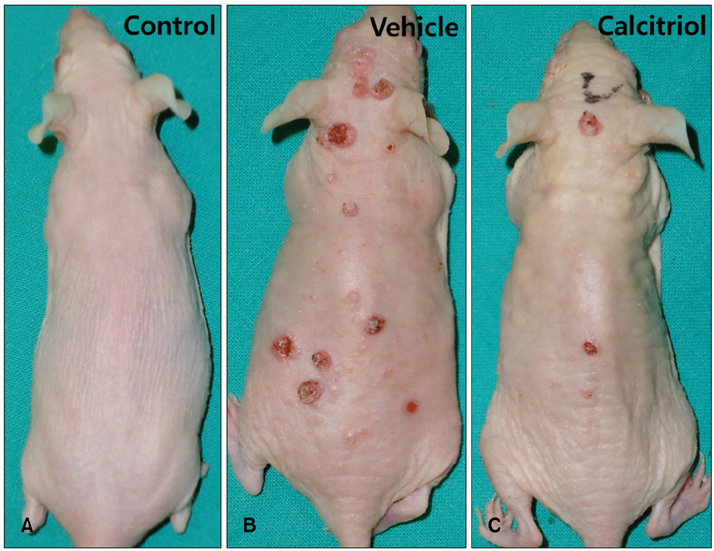
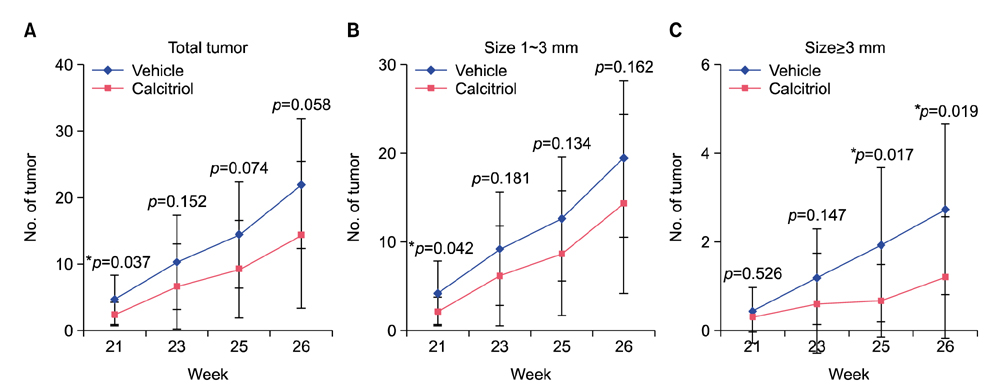
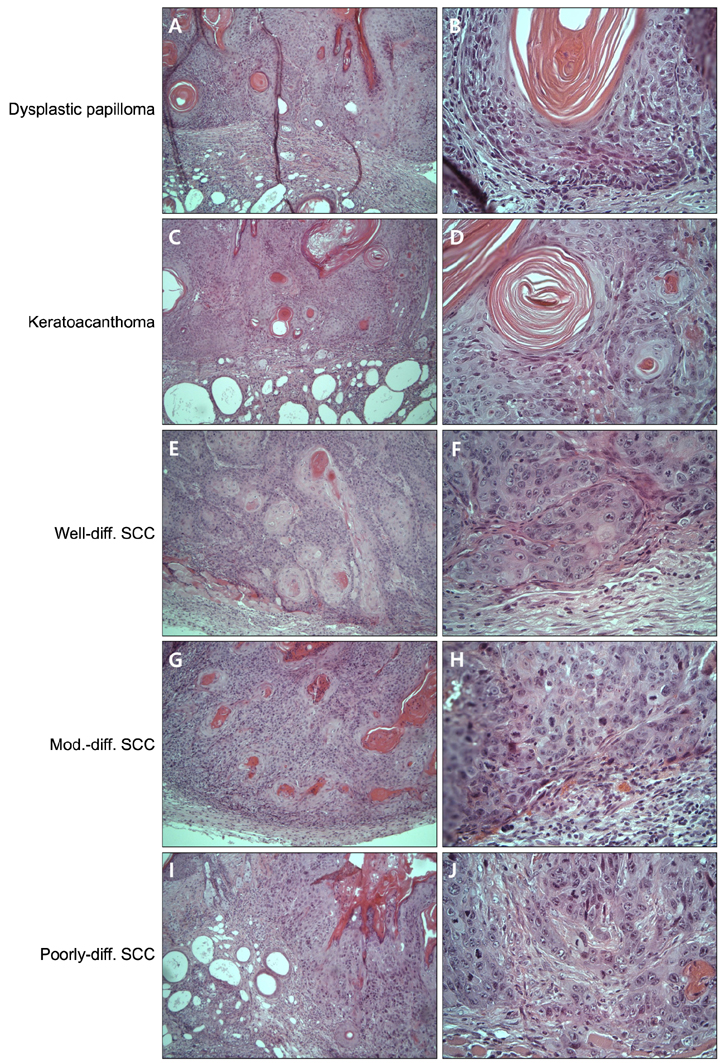
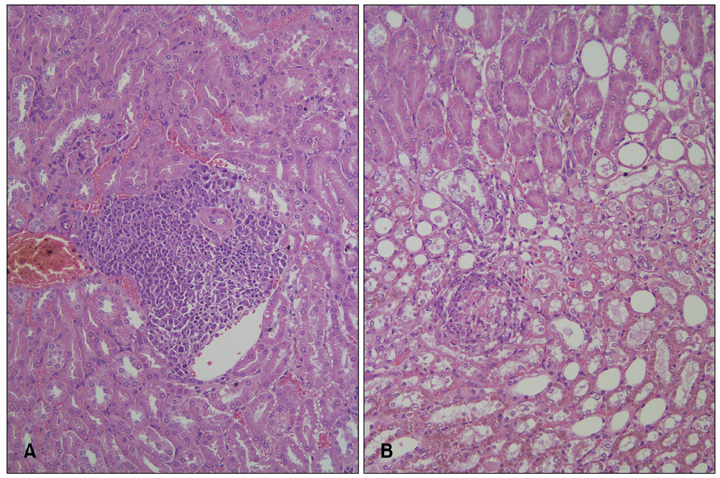
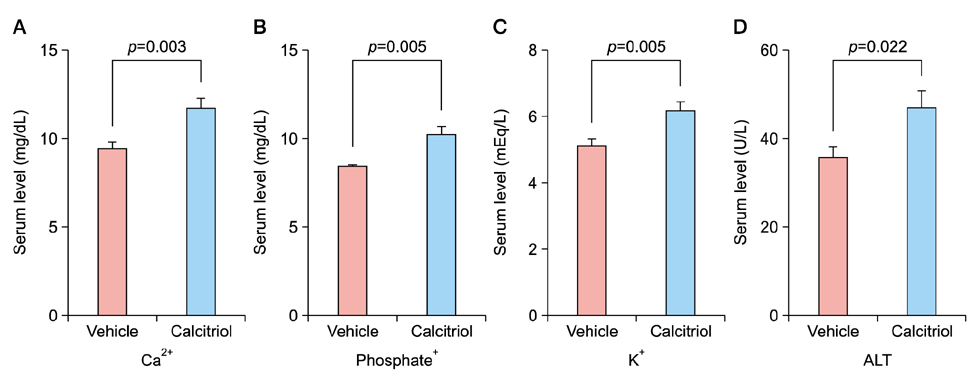
Total tumor count and number of tumors less than 3 mm in size tended to be fewer in calcitriol group, and tumors more than 3 mm in size showed significantly lower tumor formation rate in calcitriol group. Single application of calcitriol reduced CPD at 1 hour and 11 hours after UV exposure. Histopathologic analysis showed tumors with lower grade malignancy in calcitriol group which suggested a delay in tumor progression. However, serum levels of calcium and phosphate in calcitriol group were above normal range, and weight loss was found.
CONCLUSION
Topical calcitriol may suppress the formation and progression of UV-induced non-melanoma skin cancer by enhancing the repair mechanism of UV damage.
MeSH Terms
Figure
Reference
-
1. Bikle DD. Vitamin D and the skin. J Bone Miner Metab. 2010; 28:117–130.
Article2. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev. 2012; 33:456–492.
Article3. Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, et al. Biopositive effects of low-dose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008; 128:2880–2887.
Article4. Bikle DD. Protective actions of vitamin D in UVB induced skin cancer. Photochem Photobiol Sci. 2012; 11:1808–1816.
Article5. Bikle DD, Elalieh H, Welsh J, Oh D, Cleaver J, Teichert A. Protective role of vitamin D signaling in skin cancer formation. J Steroid Biochem Mol Biol. 2013; 136:271–279.
Article6. Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979; 2:1335–1336.7. Giovannucci E. Vitamin D status and cancer incidence and mortality. Adv Exp Med Biol. 2008; 624:31–42.
Article8. Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis: part I. J Am Acad Dermatol. 2012; 67:803.e1–803.e12. quiz 815-816.9. Lerche CM, Philipsen PA, Poulsen T, Wulf HC. Topical hydrocortisone, clobetasol propionate, and calcipotriol do not increase photocarcinogenesis induced by simulated solar irradiation in hairless mice. Exp Dermatol. 2010; 19:973–979.
Article10. Pommergaard HC, Burcharth J, Rosenberg J, Raskov H. Topical treatment with diclofenac, calcipotriol (vitamin-D3 analog) and difluoromethylornithine (DFMO) does not prevent nonmelanoma skin cancer in mice. Cancer Invest. 2013; 31:92–96.
Article11. Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009; 4:1350–1362.
Article12. Kusewitt DF, Applegate LA, Ley RD. Ultraviolet radiation-induced skin tumors in a south american opossum (monodelphis domestica). Vet Pathol. 1991; 28:55–65.
Article13. Gye J, Ahn SK, Kwon JE, Hong SP. Use of fractional CO2 laser decreases the risk of skin cancer development during ultraviolet exposure in hairless mice. Dermatol Surg. 2015; 41:378–386.
Article14. Demetriou SK, Ona-Vu K, Teichert AE, Cleaver JE, Bikle DD, Oh DH. Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes. J Invest Dermatol. 2012; 132:2097–2100.
Article15. Rochette PJ, Therrien JP, Drouin R, Perdiz D, Bastien N, Drobetsky EA, et al. UVA-induced cyclobutane pyrimidine dimers form predominantly at thymine-thymine dipyrimidines and correlate with the mutation spectrum in rodent cells. Nucleic Acids Res. 2003; 31:2786–2794.
Article16. Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, et al. Skin cancer prevention: a possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005; 97:137–143.
Article17. Ellison TI, Smith MK, Gilliam AC, MacDonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008; 128:2508–2517.
Article18. Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Overexpression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011; 131:2289–2297.
Article19. Mason RS, Sequeira VB, Dixon KM, Gordon-Thomson C, Pobre K, Dilley A, et al. Photoprotection by 1alpha, 25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol. 2010; 121:164–168.
Article20. Quigley DA, To MD, Perez-Losada J, Pelorosso FG, Mao JH, Nagase H, et al. Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature. 2009; 458:505–508.
Article21. Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, et al. Vitamin D3 inhibits hedgehog signaling and proliferation in murine basal cell carcinomas. Cancer Prev Res (Phila). 2011; 4:744–751.
Article22. Dixon KM, Norman AW, Sequeira VB, Mohan R, Rybchyn MS, Reeve VE, et al. 1α,25(OH)2-vitagmin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev Res (Phila). 2011; 4:1485–1494.
Article23. Kensler TW, Dolan PM, Gange SJ, Lee JK, Wang Q, Posner GH. Conceptually new deltanoids (vitamin D analogs) inhibit multistage skin tumorigenesis. Carcinogenesis. 2000; 21:1341–1345.
Article24. Wood AW, Chang RL, Huang MT, Uskokovic M, Conney AH. 1 alpha, 25-dihydroxyvitamin D3 inhibits phorbol ester-dependent chemical carcinogenesis in mouse skin. Biochem Biophys Res Commun. 1983; 116:605–611.
Article25. Burns EM, Elmets CA, Yusuf N. Vitamin D and skin cancer. Photochem Photobiol. 2015; 91:201–209.
Article26. Bikle DD, Oda Y, Tu CL, Jiang Y. Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. J Steroid Biochem Mol Biol. 2014; 148:47–51.
Article27. Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, et al. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol. 2007; 103:451–456.
Article28. Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988; 91:593–598.
Article29. Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009; 3:71–79.30. Han TY, Kong TS, Kim MH, Chae JD, Lee JH, Son SJ. Vitamin D status and its association with the SCORAD score and serum LL-37 level in korean adults and children with atopic dermatitis. Ann Dermatol. 2015; 27:10–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma
- The effect of Vitamin D(3) and TGF-beta on the viability of human periodontal ligament cells
- Successful Treatment of Alopecia Areata with Topical Calcipotriol
- Exploring optimal supplementation for people with vitamin D deficiency
- Vitamin D Deficiency and Cognitive Dysfunction