Immune Netw.
2011 Dec;11(6):399-405. 10.4110/in.2011.11.6.399.
Maturation-Resistant Dendritic Cells Ameliorate Experimental Autoimmune Uveoretinitis
- Affiliations
-
- 1Laboratory of Immunology, Transplantation Research Institute, Seoul National University College of Medicine, Seoul 110-799, Korea. dlee5522@snu.ac.kr
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul 110-799, Korea.
- KMID: 2150726
- DOI: http://doi.org/10.4110/in.2011.11.6.399
Abstract
- BACKGROUND
Endogenous uveitis is a chronic inflammatory eye disease of human, which frequently leads to blindness. Experimental autoimmune uveoretinitis (EAU) is an animal disease model of human endogenous uveitis and can be induced in susceptible animals by immunization with retinal antigens. EAU resembles the key immunological characteristics of human disease in that both are CD4+ T-cell mediated diseases. Dendritic cells (DCs) are specialized antigen-presenting cells that are uniquely capable of activating naive T cells. Regulation of immune responses through modulation of DCs has thus been tried extensively. Recently our group reported that donor strain-derived immature DC pretreatment successfully controlled the adverse immune response during allogeneic transplantation.
METHODS
EAU was induced by immunization with human interphotoreceptor retinoid-binding protein (IRBP) peptide(1-20). Dendritic cells were differentiated from bone marrow in the presence of recombinant GM-CSF.
RESULTS
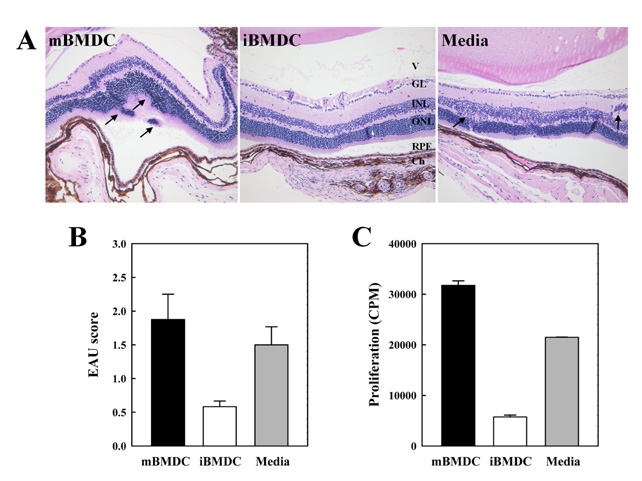
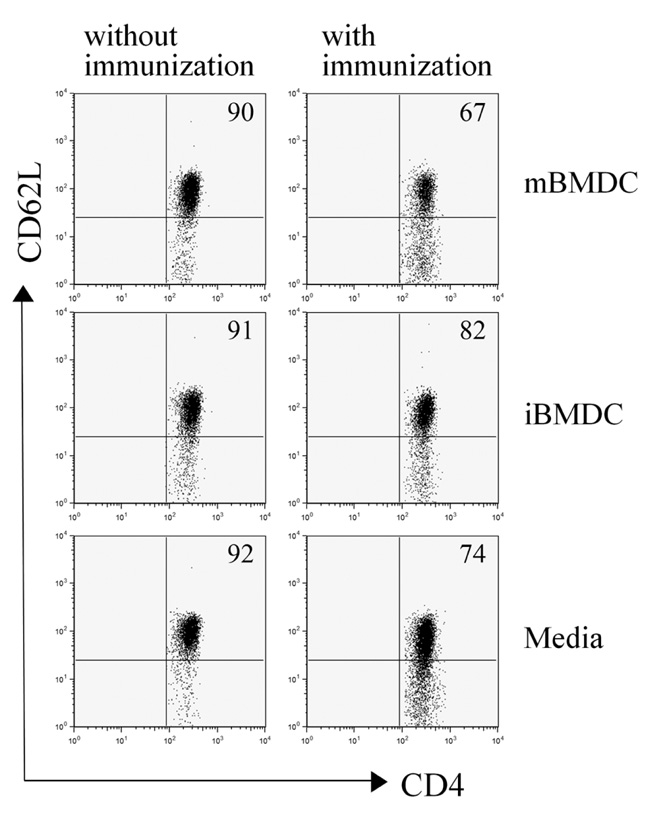
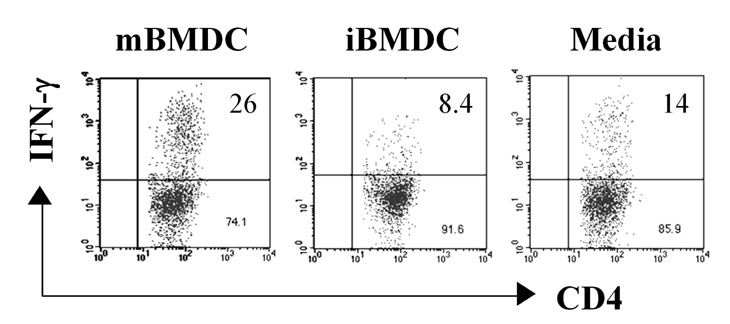
In this study, we used paraformaldehyde-fixed bone marrow-derived DCs to maintain them in an immature state. Pretreatment with fixed immature DCs, but not fixed mature DCs, ameliorated the disease progression of EAU by inhibiting uveitogenic CD4+ T cell activation and differentiation.
CONCLUSION
Application of iBMDC prepared according to the protocol of this study would provide an important treatment modality for the autoimmune diseases and transplantation rejection.
MeSH Terms
-
Animals
Antigen-Presenting Cells
Autoimmune Diseases
Blindness
Bone Marrow
Dendritic Cells
Disease Models, Animal
Disease Progression
Eye Diseases
Eye Proteins
Graft Rejection
Humans
Immunization
Retinaldehyde
Retinol-Binding Proteins
T-Lymphocytes
Tissue Donors
Uveitis
Eye Proteins
Retinaldehyde
Retinol-Binding Proteins
Figure
Reference
-
1. Ahn JK, Chung H, Lee DS, Yu YS, Yu HG. CD8brightCD56+ T cells are cytotoxic effectors in patients with active Behcet's uveitis. J Immunol. 2005. 175:6133–6142.
Article2. Yu HG, Lee DS, Seo JM, Ahn JK, Yu YS, Lee WJ, Chung H. The number of CD8+ T cells and NKT cells increases in the aqueous humor of patients with Behçet's uveitis. Clin Exp Immunol. 2004. 137:437–443.
Article3. Caspi RR, Chan CC, Wiggert B, Chader GJ. The mouse as a model of experimental autoimmune uveoretinitis (EAU). Curr Eye Res. 1990. 9:Suppl. 169–174.
Article4. Nussenblatt RB, Caspi RR, Mahdi R, Chan CC, Roberge F, Lider O, Weiner HL. Inhibition of S-antigen induced experimental autoimmune uveoretinitis by oral induction of tolerance with S-antigen. J Immunol. 1990. 144:1689–1695.5. Sun B, Rizzo LV, Sun SH, Chan CC, Wiggert B, Wilder RL, Caspi RR. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J Immunol. 1997. 159:1004–1011.6. Turley SJ. Dendritic cells: inciting and inhibiting autoimmunity. Curr Opin Immunol. 2002. 14:765–770.
Article7. O'Connell PJ, Li W, Wang Z, Specht SM, Logar AJ, Thomson AW. Immature and mature CD8alpha+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002. 168:143–154.8. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003. 21:685–711.
Article9. Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003. 18:605–617.
Article10. Adorini L. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting autoimmune diabetes. Ann N Y Acad Sci. 2003. 987:258–261.
Article11. Roelen DL, Schuurhuis DH, van den Boogaardt DE, Koekkoek K, van Miert PP, van Schip JJ, Laban S, Rea D, Melief CJ, Offringa R, Ossendorp F, Claas FH. Prolongation of skin graft survival by modulation of the alloimmune response with alternatively activated dendritic cells. Transplantation. 2003. 76:1608–1615.
Article12. Raimondi G, Thomson AW. Dendritic cells, tolerance and therapy of organ allograft rejection. Contrib Nephrol. 2005. 146:105–120.13. Kang HG, Lee JE, Yang SH, Lee SH, Gao W, Strom TB, Oh K, Lee DS, Kim YS. Donor-strain-derived immature dendritic cell pre-treatment induced hyporesponsiveness against allogeneic antigens. Immunology. 2010. 129:567–577.
Article14. Oh K, Byoun OJ, Ham DI, Kim YS, Lee DS. Invariant NKT cells regulate experimental autoimmune uveitis through inhibition of Th17 differentiation. Eur J Immunol. 2011. 41:392–402.
Article15. Chan CC, Caspi RR, Ni M, Leake WC, Wiggert B, Chader GJ, Nussenblatt RB. Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun. 1990. 3:247–255.
Article16. Sun B, Rizzo LV, Sun SH, Chan CC, Wiggert B, Wilder RL, Caspi RR. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J Immunol. 1997. 159:1004–1011.17. Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007. 13:711–718.
Article18. Tang J, Zhu W, Silver PB, Su SB, Chan CC, Caspi RR. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol. 2007. 178:5578–5587.
Article19. Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008. 205:799–810.
Article20. Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001. 167:1945–1953.
Article21. Hayamizu K, Huie P, Sibley RK, Strober S. Monocyte-derived dendritic cell precursors facilitate tolerance to heart allografts after total lymphoid irradiation. Transplantation. 1998. 66:1285–1291.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- S Antigen Specific Rat Helper T Cell Line Induced Experimental Autoimmune Uveoretinitis
- The Effects of Maturation Resistant Donor Dendritic Cells on Alloimmune Response in Mice
- Increased Expression of Intracellular HLA-DM but Not on the Surface of Blood Monocyte-derived Dendritic Cells During Maturation
- Defects in the differentiation and function of bone marrow-derived dendritic cells in non-obese diabetic mice
- Vitamin D and Immune Responses