Immune Netw.
2011 Dec;11(6):342-347. 10.4110/in.2011.11.6.342.
Modulation of Glial and Neuronal Migration by Lipocalin-2 in Zebrafish
- Affiliations
-
- 1Department of Medical Science, Korea University Ansan Hospital, Ansan 425-707, Korea.
- 2Department of Pharmacology, Brain Science & Engineering Institute, CMRI, Kyungpook National University School of Medicine, Daegu 702-701, Korea. ksuk@knu.ac.kr
- 3School of Life Sciences and Biotechnology, Kyungpook National University, Daegu 702-701, Korea.
- 4Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 136-710, Korea.
- KMID: 2150718
- DOI: http://doi.org/10.4110/in.2011.11.6.342
Abstract
- BACKGROUND
Glial cells are involved in immune and inflammatory responses in the central nervous system (CNS). Glial cells such as microglia and astrocytes also provide structural and functional support for neurons. Migration and morphological changes of CNS cells are associated with their physiological as well as pathological functions. The secreted protein lipocalin-2 (LCN2) has been previously implicated in regulation of diverse cellular processes of glia and neurons, including cell migration and morphology.
METHODS
Here, we employed a zebrafish model to analyze the role of LCN2 in CNS cell migration and morphology in vivo. In the first part of this study, we examined the indirect effect of LCN2 on cell migration and morphology of microglia, astrocytes, and neurons cultured in vitro.
RESULTS
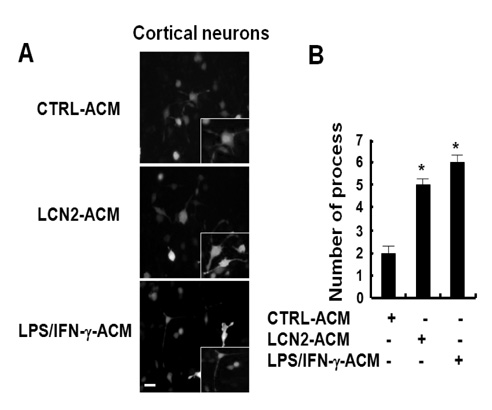
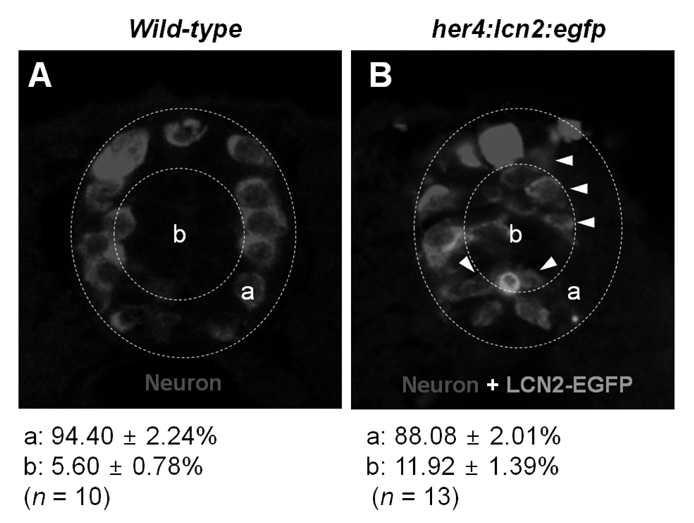
Conditioned media collected from LCN2-treated astrocytes augmented migration of glia and neurons in the Boyden chamber assay. The conditioned media also increased the number of neuronal processes. Next, in order to further understand the role of LCN2 in the CNS in vivo, LCN2 was ectopically expressed in the zebrafish spinal cord. Expression of exogenous LCN2 modulated neuronal cell migration in the spinal cord of zebrafish embryos, supporting the role of LCN2 as a cell migration regulator in the CNS.
CONCLUSION
Thus, LCN2 proteins secreted under diverse conditions may play an important role in CNS immune and inflammatory responses by controlling cell migration and morphology.
Keyword
MeSH Terms
Figure
Reference
-
1. Garden GA, Möller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006. 1:127–137.
Article2. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007. 10:1387–1394.
Article3. Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009. 27:119–145.
Article4. Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005. 123:1293–1305.
Article5. Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008. 118:1468–1478.
Article6. Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS, Kehrer JP. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005. 391:441–448.
Article7. Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009. 106:3913–3918.
Article8. Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008. 108:389–397.
Article9. Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002. 10:1045–1056.
Article10. Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008. 52:595–605.
Article11. Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004. 432:917–921.
Article12. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005. 115:610–621.
Article13. Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007. 56:2533–2540.
Article14. Lee S, Kim JH, Kim JH, Seo JW, Han HS, Lee WH, Mori K, Nakao K, Barasch J, Suk K. Lipocalin-2 is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem. 2011. 286:43855–43870.15. Lee S, Lee J, Kim S, Park JY, Lee WH, Mori K, Kim SH, Kim IK, Suk K. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007. 179:3231–3241.
Article16. Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009. 29:234–249.
Article17. Enokido Y, Akaneya Y, Niinobe M, Mikoshiba K, Hatanaka H. Basic fibroblast growth factor rescues CNS neurons from cell death caused by high oxygen atmosphere in culture. Brain Res. 1992. 599:261–271.
Article18. Araki W, Yuasa K, Takeda S, Shirotani K, Takahashi K, Tabira T. Overexpression of presenilin-2 enhances apoptotic death of cultured cortical neurons. Ann N Y Acad Sci. 2000. 920:241–244.
Article19. Chou SY, Weng JY, Lai HL, Liao F, Sun SH, Tu PH, Dickson DW, Chern Y. Expanded-polyglutamine huntingtin protein suppresses the secretion and production of a chemokine (CCL5/RANTES) by astrocytes. J Neurosci. 2008. 28:3277–3290.
Article20. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995. 203:253–310.
Article21. Yeo SY, Kim M, Kim HS, Huh TL, Chitnis AB. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev Biol. 2007. 301:555–567.
Article22. Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007. 236:3088–3099.
Article23. Kim CH, Bae YK, Yamanaka Y, Yamashita S, Shimizu T, Fujii R, Park HC, Yeo SY, Huh TL, Hibi M, Hirano T. Overexpression of neurogenin induces ectopic expression of HuC in zebrafish. Neurosci Lett. 1997. 239:113–116.
Article24. Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M, Hirano T, Miki N, Chitnis AB, Huh TL. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Dev Biol. 2000. 227:279–293.
Article