Korean J Physiol Pharmacol.
2016 Jan;20(1):25-33. 10.4196/kjpp.2016.20.1.25.
Activation of K+ channel by 1-EBIO rescues the head and neck squamous cell carcinoma cells from Ca2+ ionophore-induced cell death
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. sjoonkim@snu.ac.kr
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 4Department of Otolaryngology, Seoul National University Hospital, Seoul 03080, Korea.
- 5Cancer Research Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- 6Chung-Ang University Red Cross College of Nursing, Seoul 06974, Korea.
- KMID: 2150470
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.25
Abstract
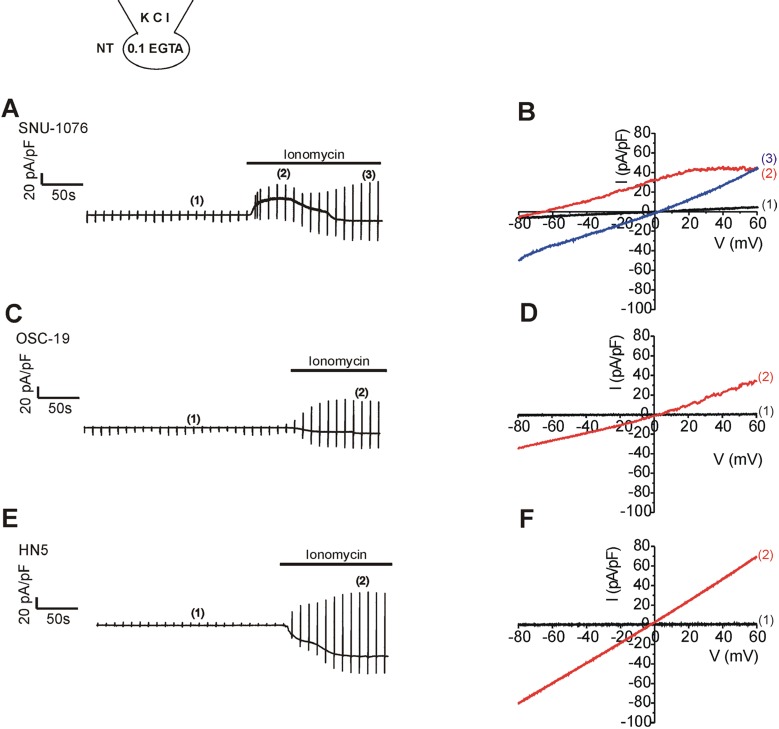
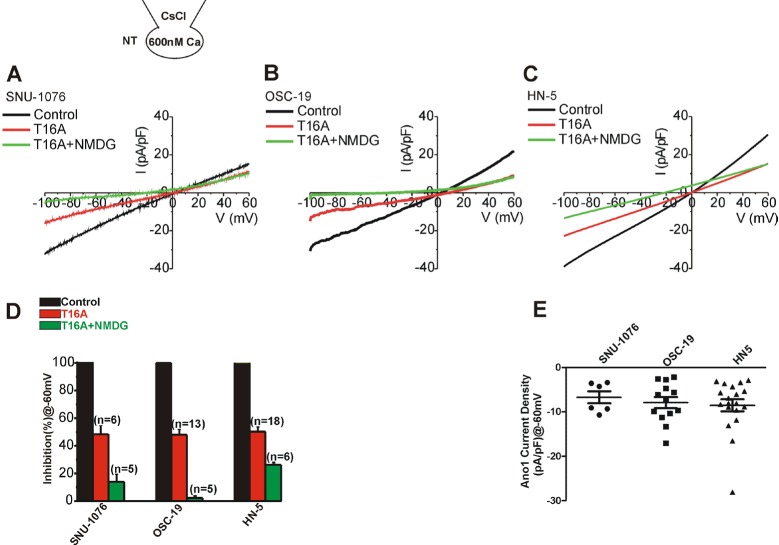
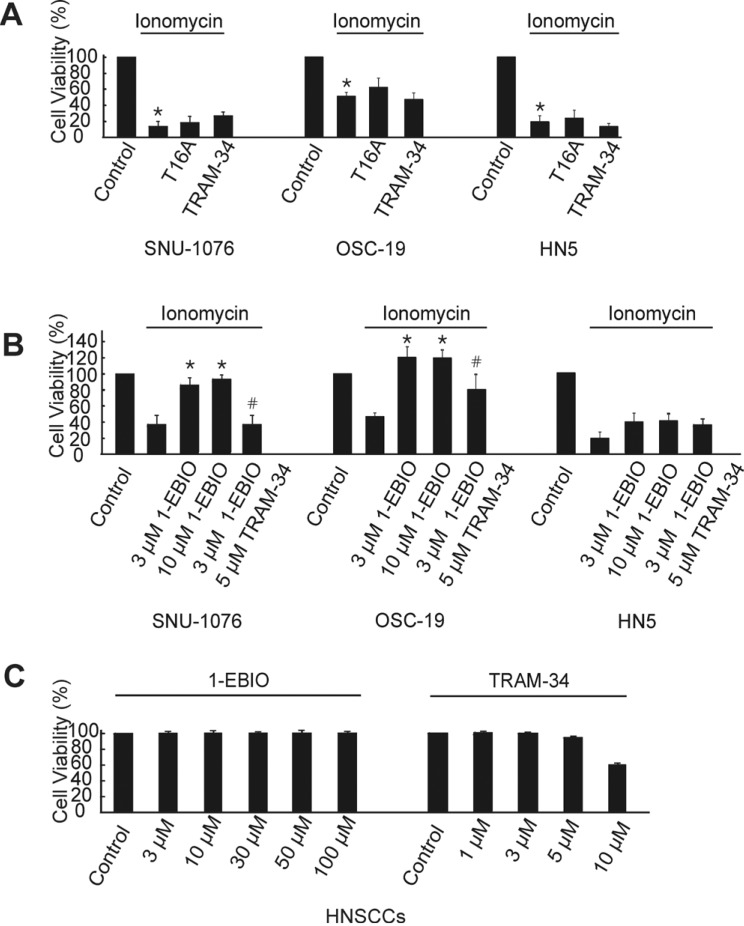
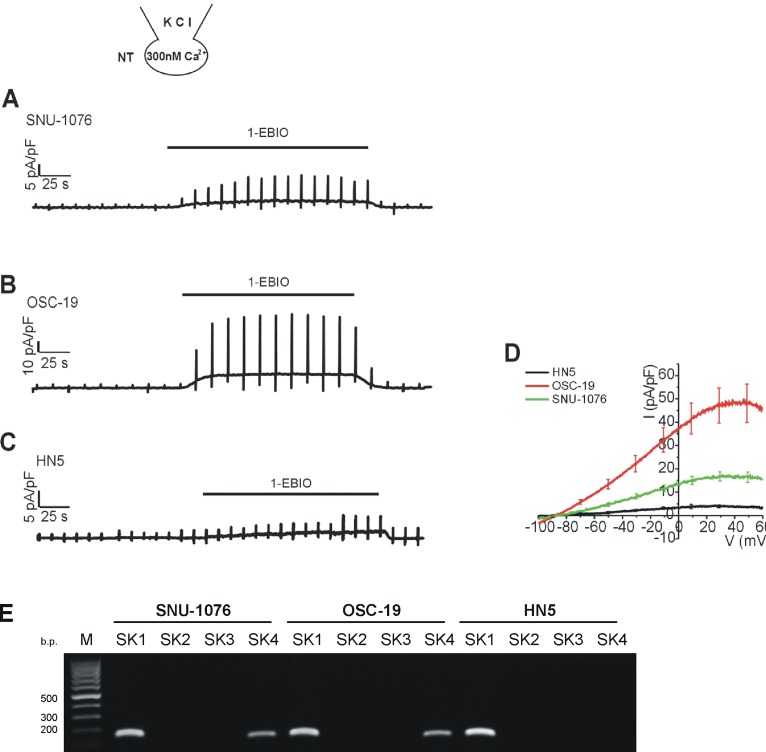
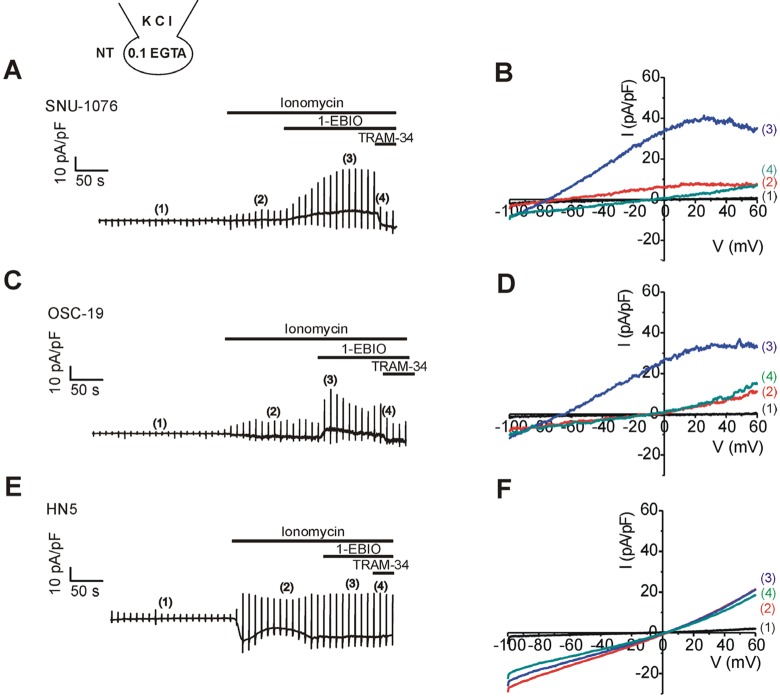
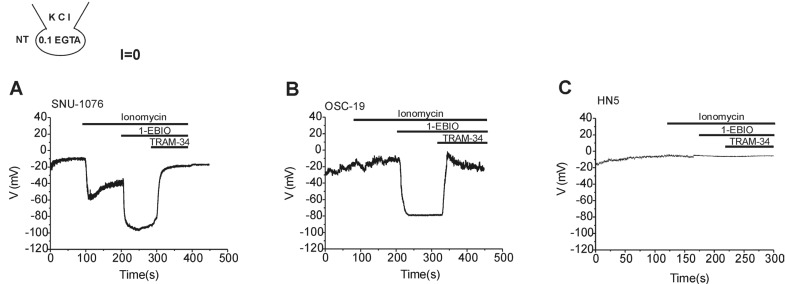
- Ion channels in carcinoma and their roles in cell proliferation are drawing attention. Intracellular Ca2+ ([Ca2+]i)-dependent signaling affects the fate of cancer cells. Here we investigate the role of Ca(2+)-activated K+ channel (SK4) in head and neck squamous cell carcinoma cells (HNSCCs) of different cell lines; SNU-1076, OSC-19 and HN5. Treatment with 1 microM ionomycin induced cell death in all the three cell lines. Whole-cell patch clamp study suggested common expressions of Ca(2+)-activated Cl- channels (Ano-1) and Ca(2+)-activated nonselective cation channels (CAN). 1-EBIO, an activator of SK4, induced outward K+ current (ISK4) in SNU-1076 and OSC-19. In HN5, ISK4 was not observed or negligible. The 1-EBIO-induced current was abolished by TRAM-34, a selective SK4 blocker. Interestingly, the ionomycin-induced cell death was effectively prevented by 1-EBIO in SNU-1076 and OSC-19, and the rescue effect was annihilated by combined TRAM-34. Consistent with the lower level of ISK4, the rescue by 1-EBIO was least effective in HN5. The results newly demonstrate the role of SK4 in the fate of HNSCCs under the Ca2+ overloaded condition. Pharmacological modulation of SK4 might provide an intriguing novel tool for the anti-cancer strategy in HNSCC.
MeSH Terms
Figure
Reference
-
1. Lim Y, Keam B, Koh Y, Kim TM, Lee SH, Hah JH, Kwon TK, Kim DW, Wu HG, Sung MW, Heo DS, Kim KH. Clinical outcomes of radiation-based locoregional therapy in locally advanced head and neck squamous cell carcinoma patients not responding to induction chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116:55–60. PMID: 23570665.
Article3. Ge L, Hoa NT, Wilson Z, Arismendi-Morillo G, Kong XT, Tajhya RB, Beeton C, Jadus MR. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int Immunopharmacol. 2014; 22:427–443. PMID: 25027630.
Article4. Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004; 5:758–770. PMID: 15378036.5. Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002; 109:397–407. PMID: 12015988.6. Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003; 100:15166–15171. PMID: 14634208.7. Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev. 2014; 94:419–459. PMID: 24692353.
Article8. Zholos A, Beck B, Sydorenko V, Lemonnier L, Bordat P, Prevarskaya N, Skryma R. Ca2+- and volume-sensitive chloride currents are differentially regulated by agonists and store- operated Ca2+ entry. J Gen Physiol. 2005; 125:197–211. PMID: 15657298.9. Park SJ, Choi WW, Kwon OS, Chung JH, Eun HC, Earm YE, Kim SJ. Acidic pH-activated Cl- current and intracellular Ca2+ response in human keratinocytes. Korean J Physiol Pharmacol. 2008; 12:177–183. PMID: 19967053.10. Ruiz C, Martins JR, Rudin F, Schneider S, Dietsche T, Fischer CA, Tornillo L, Terracciano LM, Schreiber R, Bubendorf L, Kunzelmann K. Enhanced expression of ANO1 in head and neck squamous cell carcinoma causes cell migration and correlates with poor prognosis. PLoS One. 2012; 7:e43265. PMID: 22912841.
Article11. Wanitchakool P, Wolf L, Koehl GE, Sirianant L, Schreiber R, Kulkarni S, Duvvuri U, Kunzelmann K. Role of anoctamins in cancer and apoptosis. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130096. PMID: 24493744.
Article12. Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, Schreiber R, Seethala RS, Egloff AM, Chen X, Lui VW, Grandis JR, Gollin SM. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012; 72:3270–3281. PMID: 22564524.
Article13. Girault A, Brochiero E. Evidence of K+ channel function in epithelial cell migration, proliferation, and repair. Am J Physiol Cell Physiol. 2014; 306:C307–C319. PMID: 24196531.14. Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, Larsson HP, Salathe M. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem. 2011; 286:19830–19839. PMID: 21454692.15. Takahata T, Hayashi M, Ishikawa T. SK4/IK1-like channels mediate TEA-insensitive, Ca2+-activated K+ currents in bovine parotid acinar cells. Am J Physiol Cell Physiol. 2003; 284:C127–C144. PMID: 12388063.16. Hayashi M, Wang J, Hede SE, Novak I. An intermediate-conductance Ca2+-activated K+ channel is important for secretion in pancreatic duct cells. Am J Physiol Cell Physiol. 2012; 303:C151–C159. PMID: 22555847.17. Jang Y, Oh U. Anoctamin 1 in secretory epithelia. Cell Calcium. 2014; 55:355–361. PMID: 24636668.
Article18. Shieh DB, Yang SR, Shi XY, Wu YN, Wu SN. Properties of BK(Ca) channels in oral keratinocytes. J Dent Res. 2005; 84:468–473. PMID: 15840785.
Article19. Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009; 8:3527–3536. PMID: 19823012.
Article20. Pardo LA, Stuhmer W. The roles of K+ channels in cancer. Nat Rev Cancer. 2014; 14:39–48. PMID: 24336491.21. Ernest NJ, Habela CW, Sontheimer H. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J Cell Sci. 2008; 121:290–297. PMID: 18198188.
Article22. Bortner CD, Cidlowski JA. Ion channels and apoptosis in cancer. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130104. PMID: 24493752.
Article23. Miyake H, Hara I, Yamanaka K, Arakawa S, Kamidono S. Calcium ionophore, ionomycin inhibits growth of human bladder cancer cells both in vitro and in vivo with alteration of Bcl-2 and Bax expression levels. J Urol. 1999; 162:916–921. PMID: 10458408.
Article24. Han S, Tie X, Meng L, Wang Y, Wu A. PMA and ionomycin induce glioblastoma cell death: activation-induced cell-death-like phenomena occur in glioma cells. PLoS One. 2013; 8:e76717. PMID: 24130787.
Article25. Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003; 4:552–565. PMID: 12838338.
Article26. Liu W, Lu M, Liu B, Huang Y, Wang K. Inhibition of Ca2+- activated Cl- channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012; 326:41–51. PMID: 22820160.27. Ayoub C, Wasylyk C, Li Y, Thomas E, Marisa L, Robe A, Roux M, Abecassis J, de Reynies A, Wasylyk B. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br J Cancer. 2010; 103:715–726. PMID: 20664600.
Article28. Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA. Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 2014; 369:20130094. PMID: 24493742.
Article29. Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol. 2014; 206:151–162. PMID: 25049269.
Article30. McFerrin MB, Turner KL, Cuddapah VA, Sontheimer H. Differential role of IK and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am J Physiol Cell Physiol. 2012; 303:C1070–C1078. PMID: 22992678.
Article31. Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004; 65:630–638. PMID: 14978241.32. Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004; 287:C125–C134. PMID: 14985237.33. Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol. 2003; 471:157–164. PMID: 12826234.34. Lallet-Daher H, Roudbaraki M, Bavencoffe A, Mariot P, Gackiere F, Bidaux G, Urbain R, Gosset P, Delcourt P, Fleurisse L, Slomianny C, Dewailly E, Mauroy B, Bonnal JL, Skryma R, Prevarskaya N. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene. 2009; 28:1792–1806. PMID: 19270724.35. Millership JE, Devor DC, Hamilton KL, Balut CM, Bruce JI, Fearon IM. Calcium-activated K+ channels increase cell proliferation independent of K+ conductance. Am J Physiol Cell Physiol. 2011; 300:C792–C802. PMID: 21123738.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growth Inhibition of Human Head and Neck Squamous Cell Carcinomas by Angelica decursiva Extracts
- Effects of Pharmacological Modulators of Ca2+ -activated K+ Channels on Proliferation of Human Dermal Fibroblast
- EGFR-targeted Therapy in Head and Neck Squamous Cell Carcinoma
- Herpes Viral Gene Therapy for the Treatment of Head and Neck Squamous Cell Carcinoma
- Studies of Changes of Ca2+-channels Distribution in the Activated Mouse Ova