Korean Circ J.
2013 Jul;43(7):435-442. 10.4070/kcj.2013.43.7.435.
Physiologic Assessment of Coronary Artery Disease by Cardiac Computed Tomography
- Affiliations
-
- 1Division of Cardiology, Kaiser Permanente, Panorama City, CA, USA.
- 2Cedars-Sinai Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA. james.min@cshs.org
- 3Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
- KMID: 2145498
- DOI: http://doi.org/10.4070/kcj.2013.43.7.435
Abstract
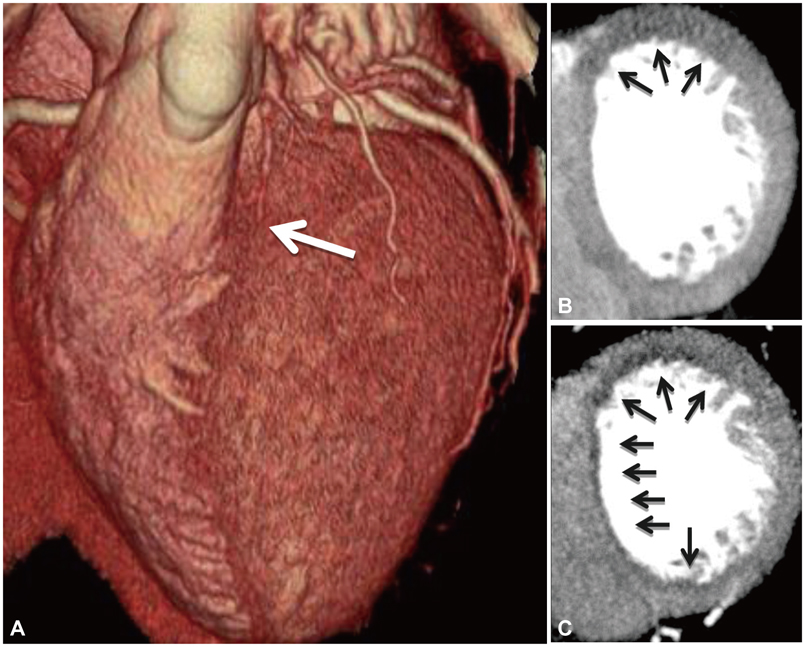
- Coronary artery disease (CAD) remains the leading cause of death and morbidity worldwide. To date, diagnostic evaluation of patients with suspected CAD has relied upon the use of physiologic non-invasive testing by stress electrocardiography, echocardiography, myocardial perfusion imaging (MPI) and magnetic resonance imaging. Indeed, the importance of physiologic evaluation of CAD has been highlighted by large-scale randomized trials that demonstrate the propitious benefit of an integrated anatomic-physiologic evaluation method by performing lesion-specific ischemia assessment by fractional flow reserve (FFR)-widely considered the "gold" standard for ischemia assessment-at the time of invasive angiography. Coronary CT angiography (CCTA) has emerged as an attractive non-invasive test for anatomic illustration of the coronary arteries and atherosclerotic plaque. In a series of prospective multicenter trials, CCTA has been proven as having high diagnostic performance for stenosis detection as compared to invasive angiography. Nevertheless, CCTA evaluation of obstructive stenoses is prone to overestimation of severity and further, detection of stenoses by CCTA does not reliably determine the hemodynamic significance of the visualized lesions. Recently, a series of technological innovations have advanced the possibility of CCTA to enable physiologic evaluation of CAD, thereby creating the potential of this test to provide an integrated anatomic-physiologic assessment of CAD. These advances include rest-stress MPI by CCTA as well as the use of computational fluid dynamics to non-invasively calculate FFR from a typically acquired CCTA. The purpose of this review is to summarize the most recent data addressing these 2 physiologic methods of CAD evaluation by CCTA.
MeSH Terms
Figure
Reference
-
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011; 123:e18–e209.2. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011; 123:933–944.3. Fischer JJ, Samady H, McPherson JA, et al. Comparison between visual assessment and quantitative angiography versus fractional flow reserve for native coronary narrowings of moderate severity. Am J Cardiol. 2002; 90:210–215.4. Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995; 92:2333–2342.5. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010; 362:886–895.6. Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol. 2010; 55:957–965.7. Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of non-invasive 64-slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006; 16:575–582.8. Raff GL, Gallagher MJ, O'Neill WW, Goldstein JA. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005; 46:552–557.9. Schuijf JD, Pundziute G, Jukema JW, et al. Diagnostic accuracy of 64-slice multislice computed tomography in the noninvasive evaluation of significant coronary artery disease. Am J Cardiol. 2006; 98:145–148.10. Ropers D, Rixe J, Anders K, et al. Usefulness of multidetector row spiral computed tomography with 64- ×0.6-mm collimation and 330-ms rotation for the noninvasive detection of significant coronary artery stenoses. Am J Cardiol. 2006; 97:343–348.11. Ehara M, Surmely JF, Kawai M, et al. Diagnostic accuracy of 64-slice computed tomography for detecting angiographically significant coronary artery stenosis in an unselected consecutive patient population: comparison with conventional invasive angiography. Circ J. 2006; 70:564–571.12. Nikolaou K, Knez A, Rist C, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR Am J Roentgenol. 2006; 187:111–117.13. Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006; 48:1896–1910.14. Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012; 308:1237–1245.15. Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008; 359:2324–2336.16. Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008; 52:2135–2144.17. Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008; 52:1724–1732.18. Mowatt G, Cummins E, Waugh N, et al. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess. 2008; 12:iii–iv. ix–143.19. Meijboom WB, Van Mieghem CA, van Pelt N, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008; 52:636–643.20. Glagov S, Bassiouny HS, Sakaguchi Y, Goudet CA, Vito RP. Mechanical determinants of plaque modeling, remodeling and disruption. Atherosclerosis. 1997; 131:Suppl. S13–S14.21. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987; 316:1371–1375.22. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009; 360:213–224.23. Pijls NH, De Bruyne B. Coronary pressure measurement and fractional flow reserve. Heart. 1998; 80:539–542.24. Elhendy A, Schinkel AF, Bax JJ, et al. Accuracy of stress Tc-99m tetrofosmin myocardial perfusion tomography for the diagnosis and localization of coronary artery disease in women. J Nucl Cardiol. 2006; 13:629–634.25. Melikian N, De Bondt P, Tonino P, et al. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv. 2010; 3:307–314.26. De Bruyne B, Baudhuin T, Melin JA, et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation. 1994; 89:1013–1022.27. Berger A, Botman KJ, MacCarthy PA, et al. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol. 2005; 46:438–442.28. Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993; 87:1354–1367.29. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012; 367:991–1001.30. Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011; 58:1989–1997.31. Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng. 2010; 38:3195–3209.32. Kim HJ, Jansen KE, Taylor CA. Incorporating autoregulatory mechanisms of the cardiovascular system in three-dimensional finite element models of arterial blood flow. Ann Biomed Eng. 2010; 38:2314–2330.33. Kim HJ, Vignon-Clementel IE, Figueroa CA, et al. On coupling a lumped parameter heart model and a three-dimensional finite element aorta model. Ann Biomed Eng. 2009; 37:2153–2169.34. Vignon-Clementel IE, Figueroa CA, Jansen KE, Taylor CA. Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries. Comput Methods Biomech Biomed Engin. 2010; 13:625–640.35. Fearon WF, Bornschein B, Tonino PA, et al. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010; 122:2545–2550.36. Pijls NH, Fearon WF, Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010; 56:177–184.37. Wolfkiel CJ, Ferguson JL, Chomka EV, et al. Measurement of myocardial blood flow by ultrafast computed tomography. Circulation. 1987; 76:1262–1273.38. George RT, Arbab-Zadeh A, Miller JM, et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009; 2:174–182.39. So A, Lee TY. Quantitative myocardial CT perfusion: a pictorial review and the current state of technology development. J Cardiovasc Comput Tomogr. 2011; 5:467–481.40. Feuchtner GM, Plank F, Pena C, et al. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart. 2012; 98:1510–1517.41. So A, Hsieh J, Narayanan S, et al. Dual-energy CT and its potential use for quantitative myocardial CT perfusion. J Cardiovasc Comput Tomogr. 2012; 6:308–317.42. Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009; 54:1072–1084.43. Feuchtner G, Goetti R, Plass A, et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ Cardiovasc Imaging. 2011; 4:540–549.44. Rocha-Filho JA, Blankstein R, Shturman LD, et al. Incremental value of adenosine-induced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology. 2010; 254:410–419.45. Williams MC, Newby DE. CT myocardial perfusion: a step towards quantification. Heart. 2012; 98:521–522.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unusual Coronary Artery Fistula: Left Anterior Descending Coronary Artery - Left Ventricular Fistula Diagnosed by ECG-Gated Multi-Detector Row Coronary CT Angiography
- Clinical Application of Cardiac Hybrid Imaging in Coronary Artery Disease
- A Single Coronary Artery with the Right Coronary Artery Originating from the Left Anterior Descending Artery Detected by Cardiac CT: A Case Report
- Non-Coronary Findings on Cardiac Computed Tomography in Adults: What Radiologists Should Know
- Current Roles and Future Applications of Cardiac CT: Risk Stratification of Coronary Artery Disease