Yonsei Med J.
2014 Jul;55(4):861-870. 10.3349/ymj.2014.55.4.861.
Insulin Receptor Expression in Clear Cell Renal Cell Carcinoma and Its Relation to Prognosis
- Affiliations
-
- 1Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea. eomm@yonsei.ac.kr
- 2Department of Occupational & Environmental Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 3Department of Urology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 4Department of Physiology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 5Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 2130808
- DOI: http://doi.org/10.3349/ymj.2014.55.4.861
Abstract
- PURPOSE
Both insulin and insulin-like growth factor (IGF)-1 signaling are key regulators of energy metabolism, cellular growth, proliferation, and survival. The IGF-1 receptor (IGF-1R) is overexpressed in most types of human cancers including renal cell carcinoma (RCC) with poor prognosis. Insulin receptor (IR) shares downstream effectors with IGF-1R; however, the expression and function of IR in the tumorigenesis of renal cancer remains elusive. Therefore, we examined the expression of IR and its prognostic significance in clear cell RCC (CCRCC).
MATERIALS AND METHODS
Immunohistochemical staining for IR was performed on 126 formalin-fixed paraffin-embedded CCRCC tissue samples. Eight of these cases were utilized for western blot analysis. The results were compared with various clinico-pathologic parameters of CCRCC and patient survival.
RESULTS
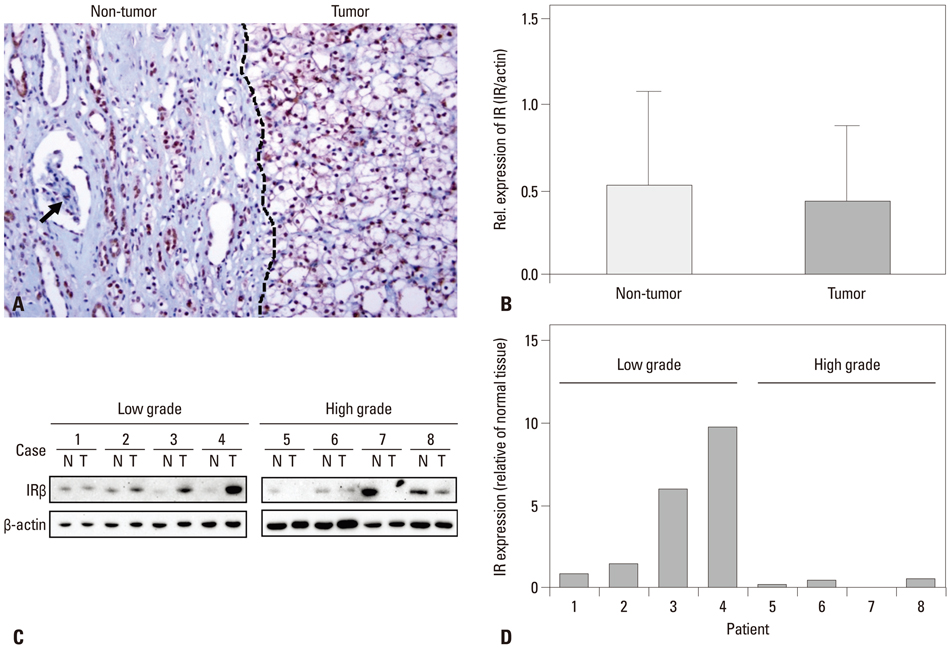
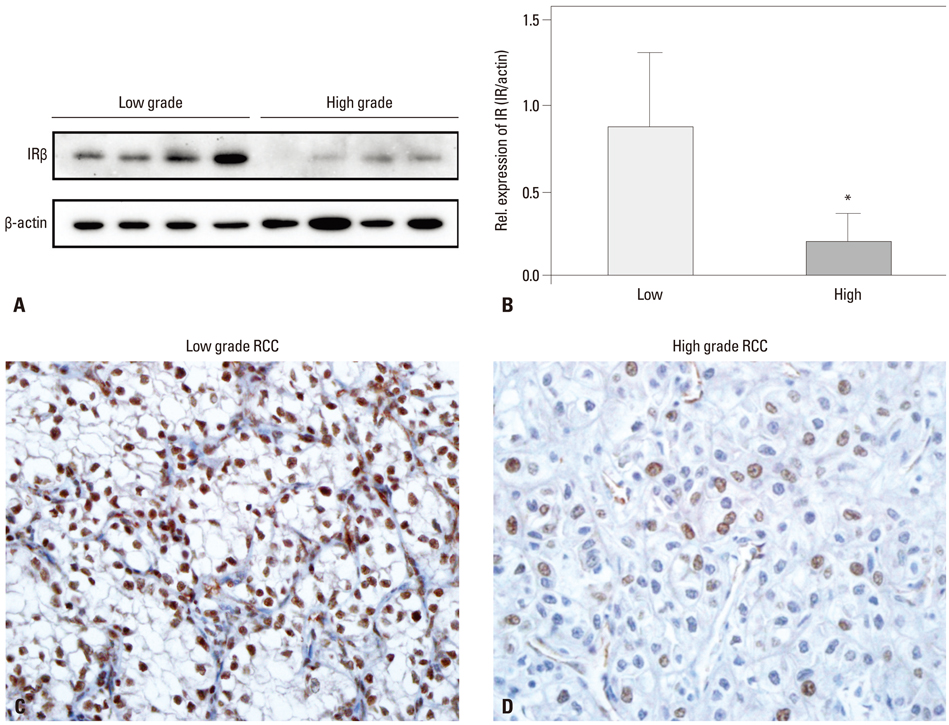
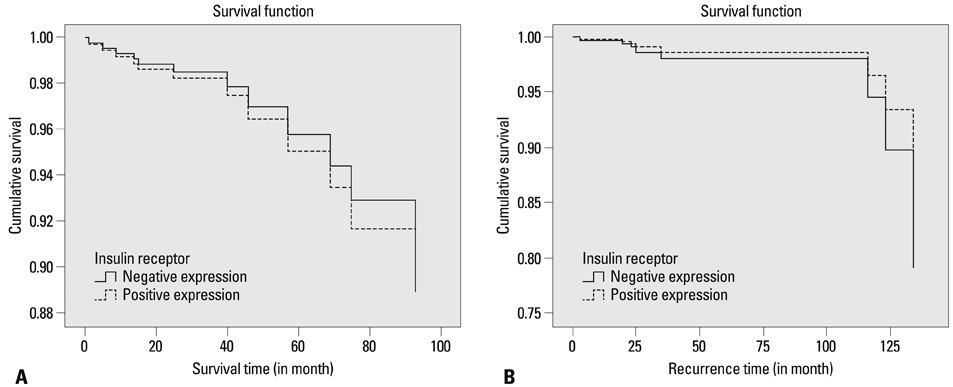
IR was expressed in the nuclei of CCRCC tumor cells in 109 cases (87.9%). Higher IR expression was significantly correlated with the presence of cystic change, lower Fuhrman nuclear grade, lower pathologic T stage, and lower TNM stage, although it wasn't significantly related to diabetes status and patient survival. Western blot analyses supported the results of the immunohistochemistry studies.
CONCLUSION
IR expression in CCRCC may be associated with favorable prognostic factors.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Klotho plays a critical role in clear cell renal cell carcinoma progression and clinical outcome
Ji-Hee Kim, Kyu-Hee Hwang, Sayamaa Lkhagvadorj, Jae Hung Jung, Hyun Chul Chung, Kyu-Sang Park, In Deok Kong, Minseob Eom, Seung-Kuy Cha
Korean J Physiol Pharmacol. 2016;20(3):297-304. doi: 10.4196/kjpp.2016.20.3.297.
Reference
-
1. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009; 373:1119–1132.
Article2. Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005; 23:2763–2771.
Article3. Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003; 30:843–852.
Article4. Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23:Suppl 7. vii65–vii71.
Article5. Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE. Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J Natl Cancer Inst. 1986; 77:351–356.6. Berry MG, Helwig FC. Marked insulin resistance in diabetes mellitus. Am J Med. 1948; 4:923–926.
Article7. Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011; 54:1013–1018.
Article8. Lindblad P, Chow WH, Chan J, Bergström A, Wolk A, Gridley G, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia. 1999; 42:107–112.
Article9. El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006; 4:369–380.
Article10. Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005; 92:2076–2083.
Article11. Xu X, Wu J, Mao Y, Zhu Y, Hu Z, Xu X, et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS One. 2013; 8:e58079.
Article12. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005; 97:1679–1687.
Article13. Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007; 121:856–862.
Article14. Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2011; 300:R1009–R1022.
Article15. Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996; 76:1005–1026.
Article16. Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000; 278:E967–E976.17. Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985; 313:756–761.
Article18. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006; 7:85–96.
Article19. Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986; 5:2503–2512.
Article20. LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003; 195:127–137.
Article21. Parker AS, Cheville JC, Janney CA, Cerhan JR. High expression levels of insulin-like growth factor-I receptor predict poor survival among women with clear-cell renal cell carcinomas. Hum Pathol. 2002; 33:801–805.
Article22. Belfiore A, Frasca F. IGF and insulin receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008; 13:381–406.
Article23. Beauchamp MC, Yasmeen A, Knafo A, Gotlieb WH. Targeting insulin and insulin-like growth factor pathways in epithelial ovarian cancer. J Oncol. 2010; 2010:257058.
Article24. Edge SB. AJCC cancer staging manual. 7th ed. New York: Springer;2010. p. 476–486.25. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982; 6:655–663.
Article26. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168.27. Ahmad N, Keehn CA, Coppola D. The expression of insulin-like growth factor-I receptor correlates with Fuhrman grading of renal cell carcinomas. Hum Pathol. 2004; 35:1132–1136.
Article28. He X, Wang J, Messing EM, Wu G. Regulation of receptor for activated C kinase 1 protein by the von Hippel-Lindau tumor suppressor in IGF-I-induced renal carcinoma cell invasiveness. Oncogene. 2011; 30:535–547.
Article29. Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010; 58:398–406.
Article30. Park HS, Jung EJ, Myung JK, Moon KC. The prognostic implications of cystic change in clear cell renal cell carcinoma. Korean J Pathol. 2010; 44:149–154.
Article31. Moller DE, Yokota A, Caro JF, Flier JS. Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol Endocrinol. 1989; 3:1263–1269.
Article32. Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, Tapscott EB, et al. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J. 1990; 270:397–400.
Article33. Höfner T, Zeier M, Hatiboglu G, Eisen C, Schönberg G, Hadaschik B, et al. The impact of type 2 diabetes on the outcome of localized renal cell carcinoma. World J Urol. 2013; [Epub ahead of print].
Article34. Parker A, Cheville JC, Lohse C, Cerhan JR, Blute ML. Expression of insulin-like growth factor I receptor and survival in patients with clear cell renal cell carcinoma. J Urol. 2003; 170(2 Pt 1):420–424.
Article35. Najjar SM, Blakesley VA, Li Calzi S, Kato H, LeRoith D, Choice CV. Differential phosphorylation of pp120 by insulin and insulin-like growth factor-1 receptors: role for the C-terminal domain of the beta-subunit. Biochemistry. 1997; 36:6827–6834.
Article36. O'Neill TJ, Zhu Y, Gustafson TA. Interaction of MAD2 with the carboxyl terminus of the insulin receptor but not with the IGFIR. Evidence for release from the insulin receptor after activation. J Biol Chem. 1997; 272:10035–10040.37. Bausch B, Jilg C, Gläsker S, Vortmeyer A, Lützen N, Anton A, et al. Renal cancer in von Hippel-Lindau disease and related syndromes. Nat Rev Nephrol. 2013; 9:529–538.
Article38. Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011; 16:Suppl 2. 4–13.
Article39. Yim S, Choi SM, Choi Y, Lee N, Chung J, Park H. Insulin and hypoxia share common target genes but not the hypoxia-inducible factor-1alpha. J Biol Chem. 2003; 278:38260–38268.
Article40. Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, et al. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010; 70:6412–6419.
Article41. Resnik JL, Reichart DB, Huey K, Webster NJ, Seely BL. Elevated insulin-like growth factor I receptor autophosphorylation and kinase activity in human breast cancer. Cancer Res. 1998; 58:1159–1164.42. Cappuzzo F, Toschi L, Tallini G, Ceresoli GL, Domenichini I, Bartolini S, et al. Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann Oncol. 2006; 17:1120–1127.
Article43. Ahlén J, Wejde J, Brosjö O, von Rosen A, Weng WH, Girnita L, et al. Insulin-like growth factor type 1 receptor expression correlates to good prognosis in highly malignant soft tissue sarcoma. Clin Cancer Res. 2005; 11:206–216.44. Sehat B, Tofigh A, Lin Y, Trocmé E, Liljedahl U, Lagergren J, et al. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Sci Signal. 2010; 3:ra10.
Article45. Amaya MJ, Oliveira AG, Guimarães ES, Casteluber MC, Carvalho SM, Andrade LM, et al. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology. 2014; 59:274–283.
Article46. Kim JS, Kim ES, Liu D, Lee JJ, Solis L, Behrens C, et al. Prognostic impact of insulin receptor expression on survival of patients with nonsmall cell lung cancer. Cancer. 2012; 118:2454–2465.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Papillary Type of Renal Cell Carcinoma after Renal Injury in a Child
- Klotho plays a critical role in clear cell renal cell carcinoma progression and clinical outcome
- The Prognostic Implications of Cystic Change in Clear Cell Renal Cell Carcinoma
- Differentiation of Chromophobe Renal Cell Carcinoma and Clear Cell Renal Cell Carcinoma by Using Helical CT
- A Case of Renal Cell Carcinoma Metastatic to the Scalp