Korean J Urol.
2007 Aug;48(8):832-837. 10.4111/kju.2007.48.8.832.
Effects of Rho Kinase Inhibitor on Detrusor Overactivity after Bladder Outlet Obstruction in Rats
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. uroljy@catholic.ac.kr
- KMID: 2061277
- DOI: http://doi.org/10.4111/kju.2007.48.8.832
Abstract
- PURPOSE
A partial bladder outlet obstruction(PBOO) related detrusor hypertrophy is associated with up-regulation of the Rho kinase activity in an experimental animal model, and has been implicated in PBOO induced bladder dysfunction. The effect of a Rho kinase inhibitor on the voiding function in anesthetized rats with PBOO was investigated.
MATERIALS AND METHODS
Eighteen Sprague-Dawley rats were divided into control(9 rats) and experimental(9 rats) groups. The experimental group was partially obstructed for 6 weeks, with cystometrograms(CMG) then were performed. The number of voids, and the intercontraction interval (ICI) and peak pressure(PP) were recorded. Rho kinase inhibitors were administered to the experimental group. An additional CMG was performed to observe the effects of Rho kinase inhibition. Bladder tissues were immunohistochemically(IHC) evaluated for the expression of RhoA protein.
RESULTS
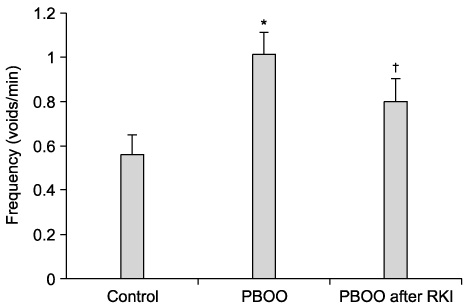
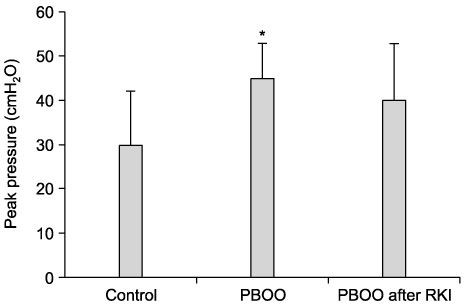
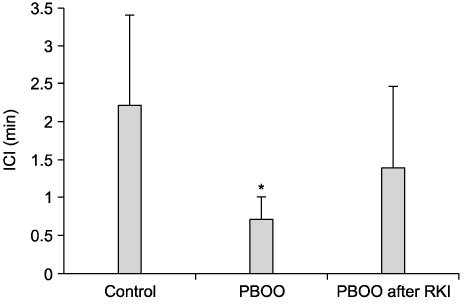
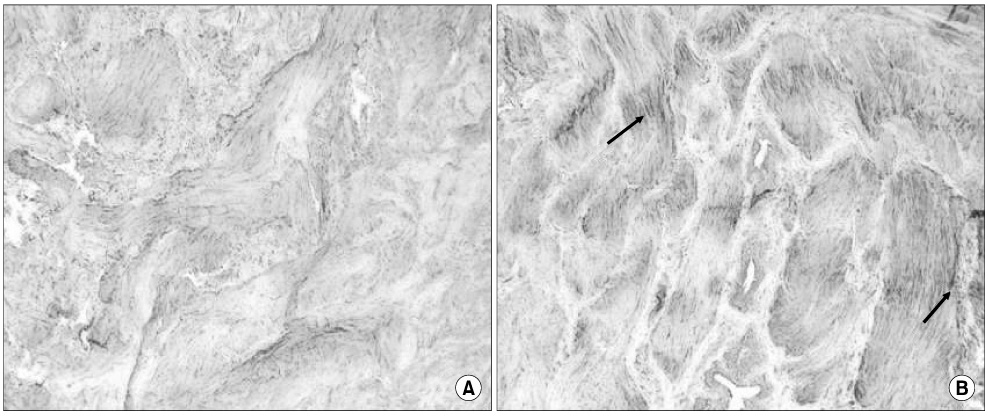
The bladder weights of the PBOO group were significantly increased compared with the control group(p<0.05). Significant increases in the voiding frequency and PP, but a significant decrease in the ICI was observed in the PBOO group compared to the control group on the CMG (p<0.05). The voiding frequency of the PBOO group was significantly decreased after Rho kinase inhibitor treatment(p<0.05). The Rho kinase inhibitor treated group showed a decrease in the PP and an increase in the ICI compared to the PBOO group. The IHC showed a higher RhoA protein expression in the bladder tissues of the PBOO group.
CONCLUSIONS
H-1152, a specific inhibitor of Rho kinase, attenuates the PBOO-related detrusor overactivity in a rat model. The Rho kinase inhibitor appears to be a novel strategy for the management of bladder overactivity.
MeSH Terms
Figure
Reference
-
1. Thomas AW, Cannon A, Bartlett E, Ellis-Jones J, Abrams P. The natural history of lower urinary tract dysfunction in men: minimum 10-year urodynamic follow-up of untreated bladder outlet obstruction. BJU Int. 2005. 96:1301–1306.2. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994. 372:231–236.3. Ogut O, Brozovich FV. Regulation of force in vascular smooth muscle. J Mol Cell Cardiol. 2003. 35:347–355.4. Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000. 522:177–185.5. Schneider T, Fetscher C, Krege S, Michel MC. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol Exp Ther. 2004. 309:1148–1153.6. Takahashi R, Nishimura J, Hirano K, Seki N, Naito S, Kanaide H. Ca2+ sensitization in contraction of human bladder smooth muscle. J Urol. 2004. 172:748–752.7. Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol. 2003. 138:757–766.8. Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, et al. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol. 2003. 285:990–997.9. Chacko S, Chang S, Hypolite J, DiSanto M, Wein A. Alteration of contractile and regulatory proteins following partial bladder outlet obstruction. Scand J Urol Nephrol Suppl. 2004. 215:26–36.10. Wilkes N, White S, Stein P, Bernie J, Rajasekaran M. Phosphodiesterase-5 inhibition synergizes rho-kinase antagonism and enhances erectile response in male hypertensive rats. Int J Impot Res. 2004. 16:187–194.11. Rajasekaran M, Kasyan A, Jain A, Kim SW, Monga M. Altered growth factor expression in the aging penis: the Brown-Norway rat model. J Androl. 2002. 23:393–399.12. Kim HS, Kim JC, Hwang TK. Effect of ACE inhibitor on connexin expression after bladder outlet obstruction in rats. Korean J Urol. 2005. 46:1205–1212.13. Malkowicz SB, Wein AJ, Elbadawi A, Van Arsdalen K, Ruggieri MR, Levin RM. Acute biochemical and functional alterations in the partially obstructed rabbit urinary bladder. J Urol. 1986. 136:1324–1329.14. Kato K, Wein AJ, Radzinski C, Longhurst PA, McGuire EJ, Miller LF, et al. Short term functional effects of bladder outlet obstruction in the cat. J Urol. 1990. 143:1020–1025.15. Kim HS, Kim JC, Hwang TK. Effect of ACE inhibitor on connexin expression after bladder outlet obstruction in rats. Korean J Urol. 2005. 46:1205–1212.16. Rajasekaran M, Wilkes N, Kuntz S, E Albo M. Rho-kinase inhibition suppresses bladder hyperactivity in spontaneously hypertensive rats. Neurourol Urodyn. 2005. 24:295–300.17. Chang S, Hypolite JA, DiSanto ME, Changolkar A, Wein AJ, Chacko S. Increased basal phosphorylation of detrusor smooth muscle myosin in alloxan-induced diabetic rabbit is mediated by upregulation of Rho-kinase beta and CPI-17. Am J Physiol Renal Physiol. 2006. 290:650–656.18. Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996. 271:20246–20249.19. Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, et al. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997. 272:12257–12260.20. Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005. 6:167–180.21. Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, et al. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002. 40:751–761.22. Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001. 7:119–122.23. Spitsbergen JM, Clemow DB, McCarty R, Steers WD, Tuttle JB. Neurally mediated hyperactive voiding in spontaneously hypertensive rats. Brain Res. 1998. 790:151–159.24. Ellenberg M. Development of urinary bladder dysfunction in diabetes mellitus. Ann Intem Med. 1980. 92:321–323.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Connexin Expression on Detrusor Overactivity in Male Patients with Bladder Outlet Obstruction Caused by Benign Prostatic Hyperplasia
- Overexpression of Aquaporin-1 and Caveolin-1 in the Rat Urinary Bladder Urothelium Following Bladder Outlet Obstruction
- The Effect of Muscarinic Receptor Subtype Antagonists on Detrusor Overactivity Induced by Bladder Outlet Obstruction in Rats
- The Clinical Significance of Detrusor Contraction Duration as a Predicting Parameter for Evaluaing Bladder Outlet Obstruction with Lower Urinary Symptoms in Men
- Effect of Bladder Outlet Obstruction on Blood Flow and Tissue Collagen in Rat Bladder