Clin Exp Vaccine Res.
2014 Jan;3(1):37-41. 10.7774/cevr.2014.3.1.37.
Prophylactic and therapeutic vaccines for obesity
- Affiliations
-
- 1Department of Infection and Obesity, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
- 2SK Chemicals, Seongnam, Korea.
- 3Department of Biotechnology, The Catholic University of Korea, Bucheon, Korea. jhnam@catholic.ac.kr
- KMID: 2049094
- DOI: http://doi.org/10.7774/cevr.2014.3.1.37
Abstract
- Chronic diseases such as obesity and diabetes are major causes of death and disability throughout the world. Many causes are known to trigger these chronic diseases, and infectious agents such as viruses are also pathological factors. In particular, it is considered that adenovirus 36 infections may be associated with obesity. If this is the case, a vaccine against adenovirus 36 may be a form of prophylaxis to combat obesity. Other types of therapeutic vaccines to combat obesity are also being developed. Recently, hormones such as glucagon-like peptide-1, ghrelin, and peptide YY have been studied as treatments to prevent obesity. This review describes the ongoing development of therapeutic vaccines to treat obesity, and the possibility of using inactivated adenovirus 36 as a vaccine and an anti-obesity agent.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. WHO World Health Report 2002: reducing risks, promoting healthy life. p. 49-97 [Internet]. Geneva: World Health Organization;2003. cited 2013 Aug 17. Available from: http://www.who.int/whr/2002/index.html.2. Rohn TA, Bachmann MF. Vaccines against non-communicable diseases. Curr Opin Immunol. 2010; 22:391–396.
Article3. World Health Organization. Fact Sheet 311: obesity and overweight [Internet]. Geneva: World Health Organization;2013. cited 2013 Aug 17. Available from: http://www.who.int/mediacentre/factsheets/fs311/en.4. Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011; 96:1654–1663.
Article5. Schuster DP. Obesity and the development of type 2 diabetes: the effects of fatty tissue inflammation. Diabetes Metab Syndr Obes. 2010; 3:253–262.
Article6. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus: present and future perspectives. Nat Rev Endocrinol. 2012; 8:228–236.
Article7. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005; 293:43–53.
Article8. Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007; 369:71–77.
Article9. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007; 357:753–761.
Article10. Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007; 357:741–752.
Article11. Melnikova I, Wages D. Anti-obesity therapies. Nat Rev Drug Discov. 2006; 5:369–370.
Article12. Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001; 86:4753–4758.
Article13. Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004; 18:439–456.
Article14. Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007; 8:21–34.
Article15. St-Pierre DH, Karelis AD, Cianflone K, et al. Relationship between ghrelin and energy expenditure in healthy young women. J Clin Endocrinol Metab. 2004; 89:5993–5997.
Article16. Zorrilla EP, Iwasaki S, Moss JA, et al. Vaccination against weight gain. Proc Natl Acad Sci U S A. 2006; 103:13226–13231.
Article17. Wilding JP. Neuropeptides and appetite control. Diabet Med. 2002; 19:619–627.
Article18. Rojas JM, Stafford JM, Saadat S, Printz RL, Beck-Sickinger AG, Niswender KD. Central nervous system neuropeptide Y signaling via the Y1 receptor partially dissociates feeding behavior from lipoprotein metabolism in lean rats. Am J Physiol Endocrinol Metab. 2012; 303:E1479–E1488.
Article19. NHS Choice. 'Obesity vaccine' hope [Internet]. Geneva: World Health Organization;2003. cited 2013 Aug 17. Available from: http://www.nhs.uk/news/2012/07July/Pages/obesity-vaccine-fat-jab-hope.aspx.20. Johnson JE, Chiu W. Structures of virus and virus-like particles. Curr Opin Struct Biol. 2000; 10:229–235.
Article21. Pumpens P, Grens E. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 1999; 442:1–6.
Article22. Kozlovska TM, Cielens I, Dreilinna D, et al. Recombinant RNA phage Q beta capsid particles synthesized and self-assembled in Escherichia coli. Gene. 1993; 137:133–137.
Article23. Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004; 303:1526–1529.
Article24. Bessa J, Jegerlehner A, Hinton HJ, et al. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J Immunol. 2009; 183:3788–3799.
Article25. Hou B, Saudan P, Ott G, et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011; 34:375–384.
Article26. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444:840–846.
Article27. Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986; 232:1545–1547.
Article28. Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010; 17:314–321.29. Bachmann MF, Jennings GT. Therapeutic vaccines for chronic diseases: successes and technical challenges. Philos Trans R Soc Lond B Biol Sci. 2011; 366:2815–2822.
Article30. Spohn G, Keller I, Beck M, Grest P, Jennings GT, Bachmann MF. Active immunization with IL-1 displayed on virus-like particles protects from autoimmune arthritis. Eur J Immunol. 2008; 38:877–887.
Article31. Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obes Rev. 2001; 2:131–140.
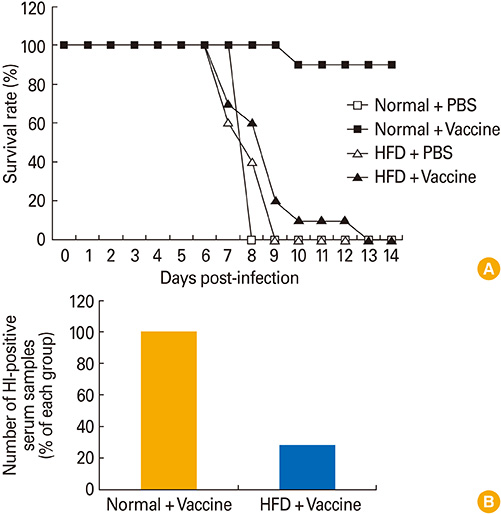
Article32. Kim YH, Kim JK, Kim DJ, et al. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. J Infect Dis. 2012; 205:244–251.
Article33. Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985; 254:3187–3189.
Article34. Young MD, Gooch WM 3rd, Zuckerman AJ, Du W, Dickson B, Maddrey WC. Comparison of a triple antigen and a single antigen recombinant vaccine for adult hepatitis B vaccination. J Med Virol. 2001; 64:290–298.
Article35. Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci U S A. 2008; 105:2017–2021.
Article36. Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord. 2000; 24:989–996.
Article37. Dhurandhar NV, Israel BA, Kolesar JM, Mayhew G, Cook ME, Atkinson RL. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord. 2001; 25:990–996.
Article38. Dhurandhar NV, Whigham LD, Abbott DH, et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002; 132:3155–3160.
Article39. van Ginneken V, Sitnyakowsky L, Jeffery JE. "Infectobesity: viral infections (especially with human adenovirus-36: Ad-36) may be a cause of obesity. Med Hypotheses. 2009; 72:383–388.
Article40. Atkinson RL, Dhurandhar NV, Allison DB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond). 2005; 29:281–286.
Article41. Na HN, Hong YM, Kim J, Kim HK, Jo I, Nam JH. Association between human adenovirus-36 and lipid disorders in Korean schoolchildren. Int J Obes (Lond). 2010; 34:89–93.
Article42. Atkinson RL, Lee I, Shin HJ, He J. Human adenovirus-36 antibody status is associated with obesity in children. Int J Pediatr Obes. 2010; 5:157–160.
Article43. Trovato GM, Castro A, Tonzuso A, et al. Human obesity relationship with Ad36 adenovirus and insulin resistance. Int J Obes (Lond). 2009; 33:1402–1409.
Article44. Gabbert C, Donohue M, Arnold J, Schwimmer JB. Adenovirus 36 and obesity in children and adolescents. Pediatrics. 2010; 126:721–726.
Article45. Pasarica M, Loiler S, Dhurandhar NV. Acute effect of infection by adipogenic human adenovirus Ad36. Arch Virol. 2008; 153:2097–2102.
Article