Anat Cell Biol.
2013 Mar;46(1):49-56. 10.5115/acb.2013.46.1.49.
Qualitative changes in fetal trabecular meshwork fibers at the human iridocorneal angle
- Affiliations
-
- 1Division of Ophthalmology, Iwamizawa Municipal Hospital, Iwamizawa, Japan. fmhosaka@gmail.com
- 2Department of Anatomy and Embryology II, Faculty of Medicine, Complutense University, Madrid, Spain.
- 3Department of Anatomy, Akita University School of Medicine, Akita, Japan.
- 4Division of Internal Medicine, Iwamizawa Kojin-kai Hospital, Iwamizawa, Japan.
- 5Department of Anatomy, Sapporo Medical University, Sapporo, Japan.
- 6Department of Ophthalmology, Sapporo Medical University, Sapporo, Japan.
- KMID: 2046757
- DOI: http://doi.org/10.5115/acb.2013.46.1.49
Abstract
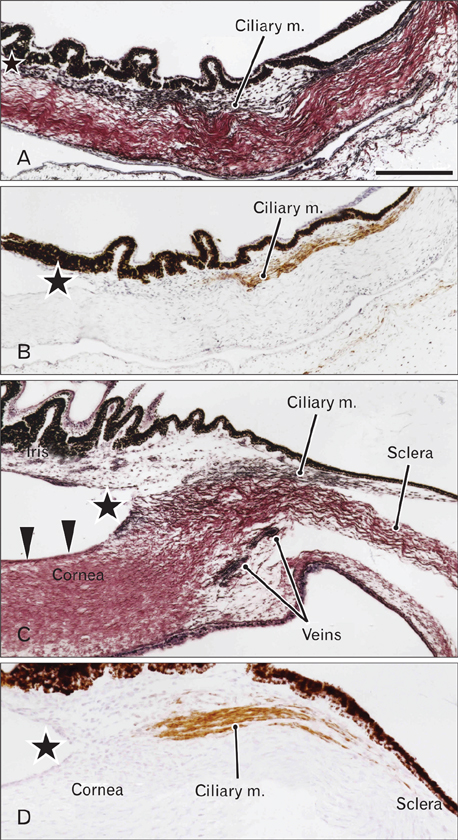
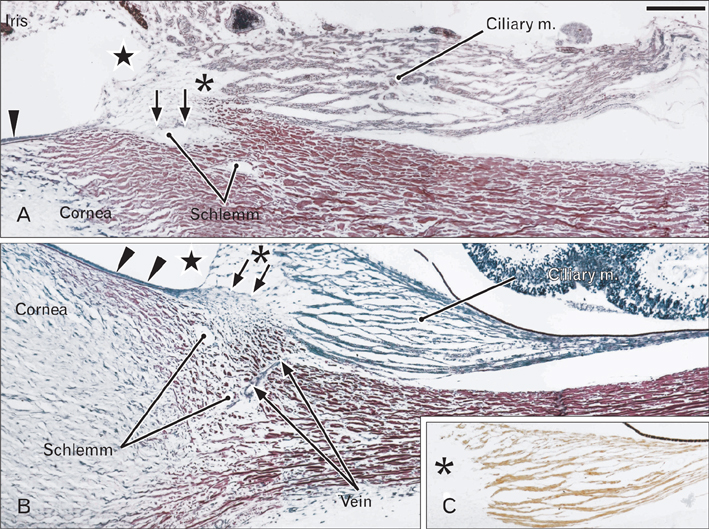
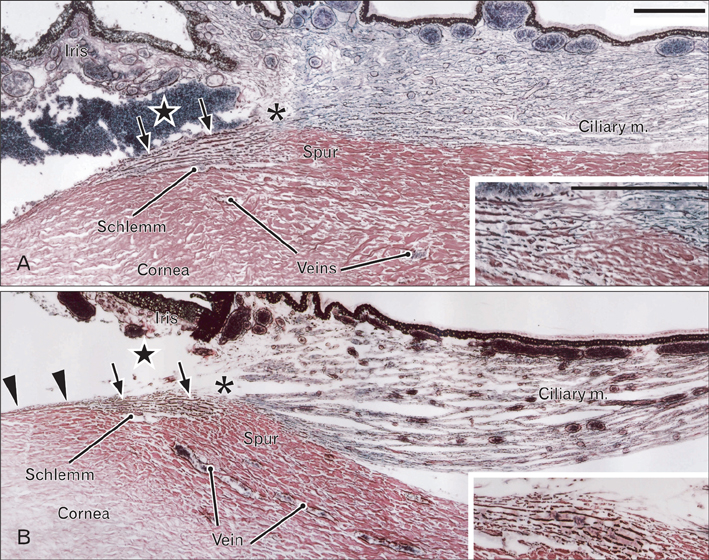
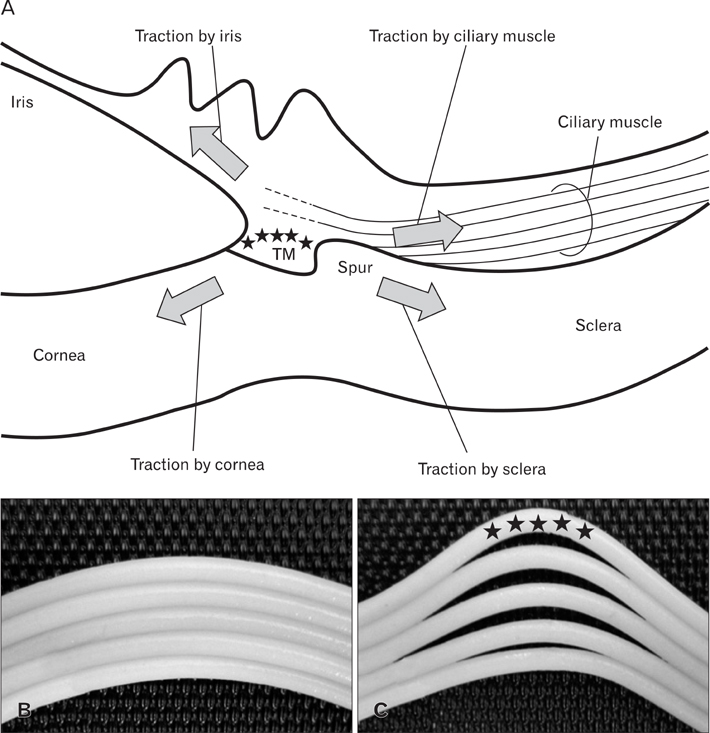
- We examined a series of changes that occur in the trabecular meshwork fibers of human eyes during fetal development at 12-30 weeks of gestation. At 12 and 15 weeks, the uveal meshwork was stained black with silver impregnation (indicating the predominance of collagen types III and IV) in the endomysium of the ciliary muscle. At 20 weeks, in combination with Schlemm's canal, a dense fibrous tissue mass corresponding to the trabecular meshwork anlage appeared and was colored black. The anlage was continuous with the corneal endothelium rather than with the ciliary muscle. Until 25 weeks, the trabecular meshwork was identifiable as fragmented fiber bundles that stained red-black, suggesting a mixture of collagen types I, III, and IV. At 30 weeks, half of the ciliary muscle fibers were inserted into the scleral spur and not into the meshwork. Therefore, any contribution of ciliary muscle contraction to the differentiation of the trabecular meshwork would appear to be limited. We hypothesize that an uneven distribution of mechanical stresses in the area of the cornea-sclera junction causes a tear thereby creating Schlemm's canal and is accompanied by a change in the collagen fiber types comprising the meshwork.
MeSH Terms
Figure
Reference
-
1. McMenamin PG. Human fetal iridocorneal angle: a light and scanning electron microscopic study. Br J Ophthalmol. 1989. 73:871–879.2. McMenamin PG. A morphological study of the inner surface of the anterior chamber angle in pre and postnatal human eyes. Curr Eye Res. 1989. 8:727–739.3. McMenamin PG. A quantitative study of the prenatal development of the aqueous outflow system in the human eye. Exp Eye Res. 1991. 53:507–517.4. VanderWyst SS, Perkumas KM, Read AT, Overby DR, Stamer WD. Structural basement membrane components and corresponding integrins in Schlemm's canal endothelia. Mol Vis. 2011. 17:199–209.5. Kupfer C. A note on the development of the anterior chamber angle. Invest Ophthalmol. 1969. 8:69–74.6. Kupfer C, Ross K. The development of outflow facility in human eyes. Invest Ophthalmol. 1971. 10:513–517.7. Anderson DR. The development of the trabecular meshwork and its abnormality in primary infantile glaucoma. Trans Am Ophthalmol Soc. 1981. 79:458–485.8. Hansson HA, Jerndal T. Scanning electron microscopic studies on the development of the iridocorneal angle in human eyes. Invest Ophthalmol. 1971. 10:252–265.9. Ruano-Gil D, Costa-Vila J, Barastegui C. Arrangement of the sclerocorneal trabecular system in human fetuses. Acta Anat (Basel). 1986. 127:233–236.10. Tripathi RC. Davson H, Graham LT, editors. Comparative physiology and anatomy of the aqueous outflow pathway in the eye. The Eye. 1974. New York: Academic Press;163–336.11. Hogan MJ, Alvarado JA, Weddell JE. Histology of the human eye: an atlas and textbook. 1971. Philadelphia: W. B. Saunders.12. Meghpara B, Li X, Nakamura H, Khan A, Bejjani BA, Lin S, Edward DP. Human anterior chamber angle development without cell death or macrophage involvement. Mol Vis. 2008. 14:2492–2498.13. Marshall GE, Konstas AG, Lee WR. Collagens in ocular tissues. Br J Ophthalmol. 1993. 77:515–524.14. Abe S, Rhee SK, Osonoi M, Nakamura T, Cho BH, Murakami G, Ide Y. Expression of intermediate filaments at muscle insertions in human fetuses. J Anat. 2010. 217:167–173.15. Osanai H, Abe S, Rodríguez-Vázquez J, Verdugo-López S, Murakami G, Ohguro H. Human orbital muscle: a new point of view from the fetal development of extraocular connective tissues. Invest Ophthalmol Vis Sci. 2011. 52:1501–1506.16. Osanai H, Rodríguez-Vázquez JF, Abe H, Murakami G, Ohguro H, Fujimiya M. Fetal check ligament connected between the conjunctiva and the medial and lateral recti. Invest Ophthalmol Vis Sci. 2011. 52:7175–7179.17. Speakman JS. Drainage channels in the trabecular wall of Schlemm's canal. Br J Ophthalmol. 1960. 44:513–523.18. Lillie RD, Tracy RE, Pizzolato P, Donaldson PT, Reynolds C. Differential staining of collagen types in paraffin sections: a color change in degraded forms. Virchows Arch A Pathol Anat Histol. 1980. 386:153–159.19. Satoh T, Takeda R, Oikawa H, Satodate R. Immunohistochemical and structural characteristics of the reticular framework of the white pulp and marginal zone in the human spleen. Anat Rec. 1997. 249:486–494.20. Yamanouchi M. An electron microscopic study of the human iris vessels with special reference to the vascular changes on aging, using PAM-stain technique. Nihon Ganka Gakkai Zasshi. 1969. 73:767–784.21. Duance VC, Stephens HR, Dunn M, Bailey AJ, Dubowitz V. A role for collagen in the pathogenesis of muscular dystrophy? Nature. 1980. 284:470–472.22. Sevel D, Isaacs R. A re-evaluation of corneal development. Trans Am Ophthalmol Soc. 1988. 86:178–207.23. Rehnberg M, Ammitzböll T, Tengroth B. Collagen distribution in the lamina cribrosa and the trabecular meshwork of the human eye. Br J Ophthalmol. 1987. 71:886–892.24. Bystrom B, Virtanen I, Rousselle P, Gullberg D, Pedrosa-Domellof F. Distribution of laminins in the developing human eye. Invest Ophthalmol Vis Sci. 2006. 47:777–785.25. Ishii T. On the light microscopic fine structure of reticular fibers in the lymph node (a contribution to the understanding of the nature of reticular fibers). Verh Anat Ges. 1967. 62:487–494.26. Lutjen-Drecoll E, Futa R, Rohen JW. Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1981. 21:563–573.27. Tamura Y, Konomi H, Sawada H, Takashima S, Nakajima A. Tissue distribution of type VIII collagen in human adult and fetal eyes. Invest Ophthalmol Vis Sci. 1991. 32:2636–2644.28. Grierson I, Rahi AH. Microfilaments in the cells of the human trabecular meshwork. Br J Ophthalmol. 1979. 63:3–8.29. Tumminia SJ, Mitton KP, Arora J, Zelenka P, Epstein DL, Russell P. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998. 39:1361–1371.30. Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2007. 48:1164–1172.31. Smelser GK, Ozanics V. The development of the trabecular meshwork in primate eyes. Am J Ophthalmol. 1971. 71(1 Pt 2):366–385.32. Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004. 13:421–438.33. Hamanaka T, Bill A, Ichinohasama R, Ishida T. Aspects of the development of Schlemm's canal. Exp Eye Res. 1992. 55:479–488.34. Smith RS, Zabaleta A, Savinova OV, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001. 1:3.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphologic Changes of Anterior Chamber Angle and Trabecular Meshwork According to Embryonic Age in Human Fetal Eyes
- Age-related Changes of the Cellularity and Acid Mucopolysaccharides in the Trabecular Meshwork of the Normal Korean
- Change of Intraocular Pressure in Trabecular Meshwork Rupture Associated with Traumatic Hyphema
- Distribution of proteoglycans in the human trabecular meshwork: histochemical electron microscopic findings with cuprolinic blue
- The Effect of Accumulation of Mutant Myocilins in Human Trabecular Meshwork Cells