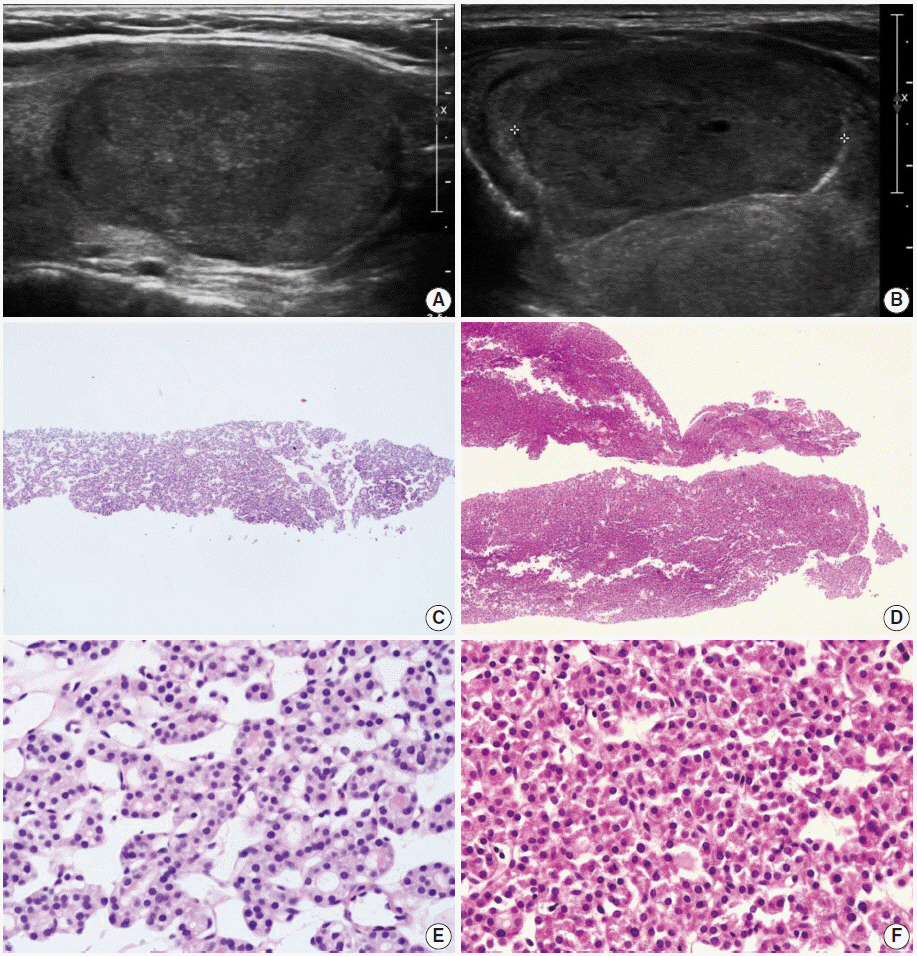
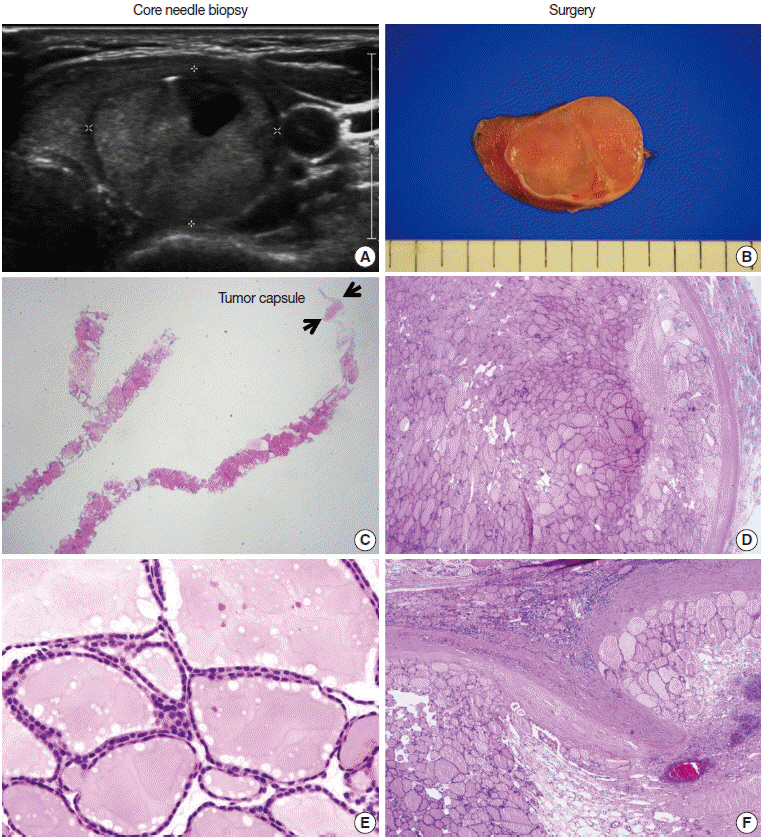
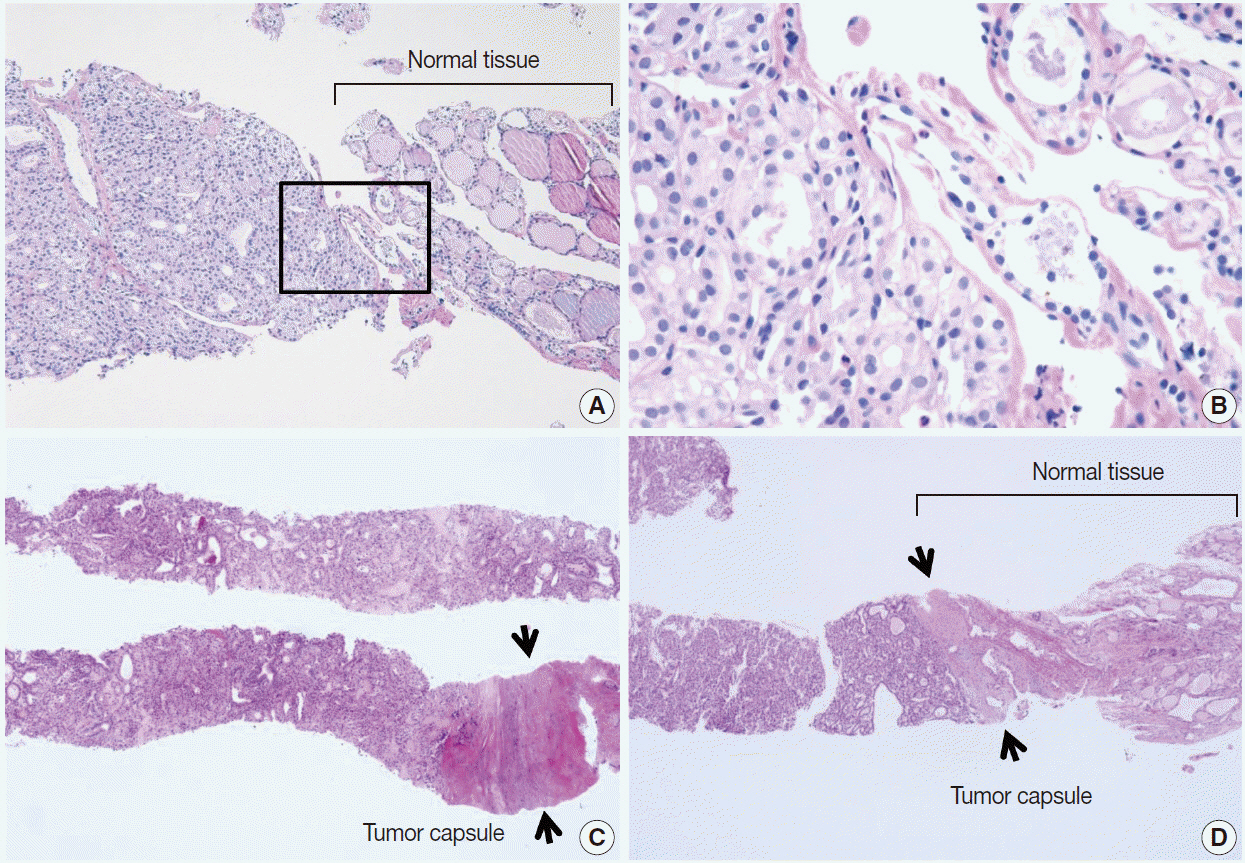
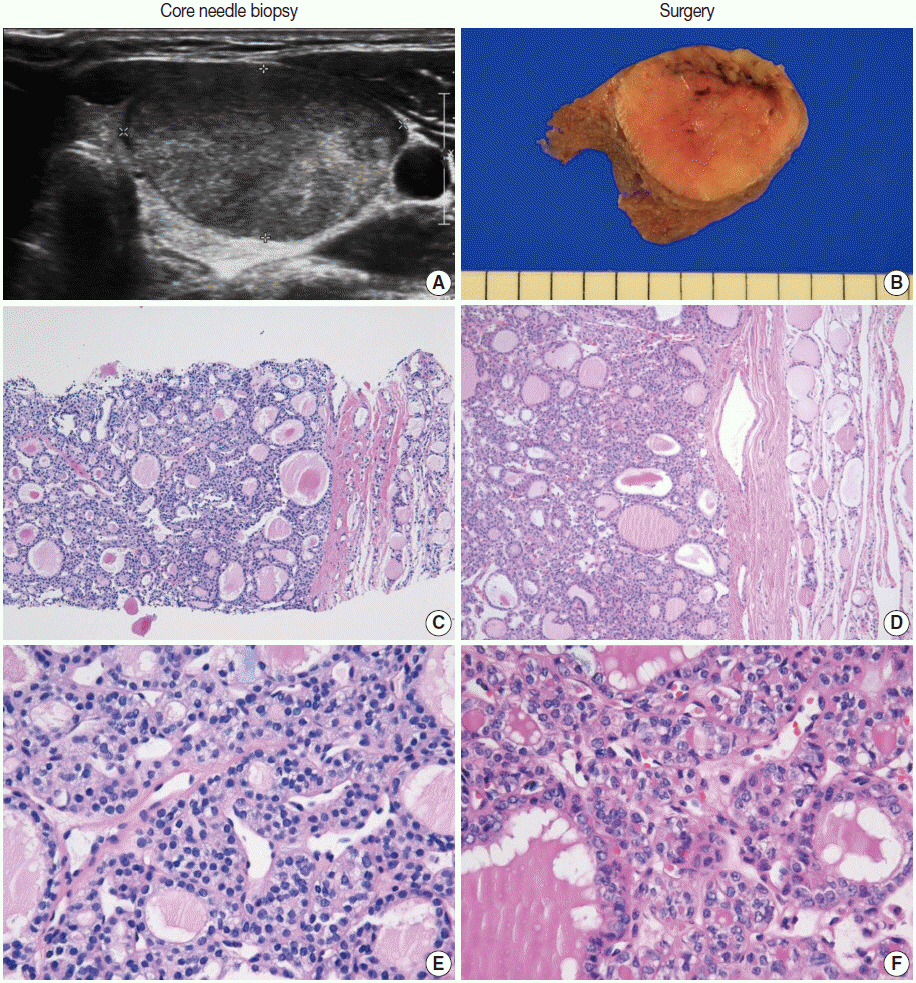
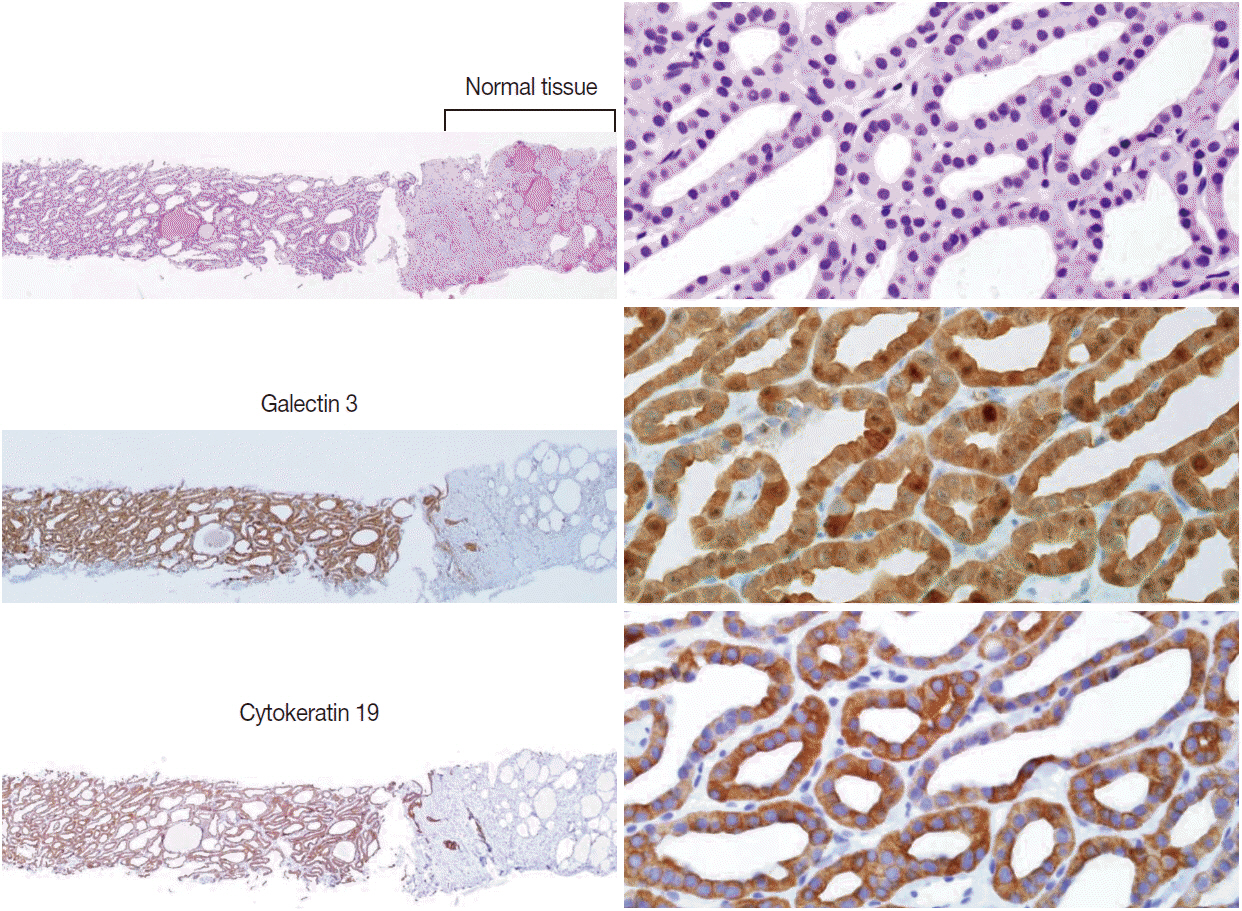
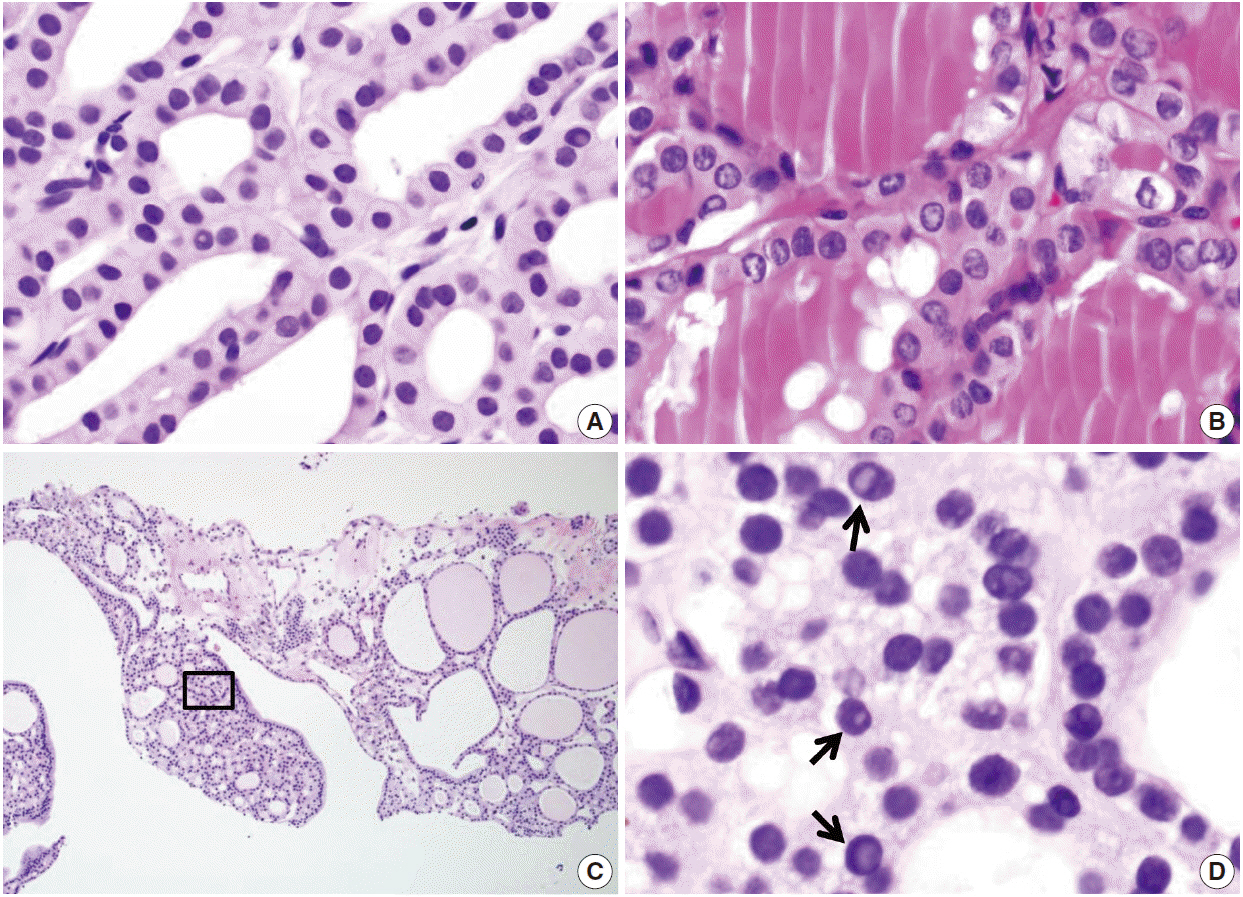
Pathology Reporting of Thyroid Core Needle Biopsy: A Proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group
- Affiliations
-
- 1Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea. ckjung@catholic.ac.kr
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Epidemiology and Preventive Medicine, Graduate School of Public Health, Seoul National University, Seoul, Korea.
- 4Department of Pathology, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
- 6Department of Pathology, Daerim Saint Mary's Hospital, Seoul, Korea.
- 7Department of Pathology, Hallym University College of Medicine, Seoul, Korea. smk0103@yahoo.co.kr
- KMID: 2151138
- DOI: http://doi.org/10.4132/jptm.2015.06.04
Abstract
- In recent years throughout Korea, the use of ultrasound-guided core needle biopsy (CNB) has become common for the preoperative diagnosis of thyroid nodules. However, there is no consensus on the pathology reporting system for thyroid CNB. The Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group held a conference on thyroid CNB pathology and developed guidelines through contributions from the participants. This article discusses the outcome of the discussions that led to a consensus on the pathology reporting of thyroid CNB.
MeSH Terms
Figure
Cited by 17 articles
-
Comparison of Immunohistochemistry and Direct Sanger Sequencing for Detection of the BRAFV600E Mutation in Thyroid Neoplasm
Hye-Seon Oh, Hyemi Kwon, Suyeon Park, Mijin Kim, Min Ji Jeon, Tae Yong Kim, Young Kee Shong, Won Bae Kim, Jene Choi, Won Gu Kim, Dong Eun Song
Endocrinol Metab. 2018;33(1):62-69. doi: 10.3803/EnM.2018.33.1.62.Concordance of Three International Guidelines for Thyroid Nodules Classified by Ultrasonography and Diagnostic Performance of Biopsy Criteria
Younghee Yim, Dong Gyu Na, Eun Ju Ha, Jung Hwan Baek, Jin Yong Sung, Ji-hoon Kim, Won-Jin Moon
Korean J Radiol. 2020;21(1):108-116. doi: 10.3348/kjr.2019.0215.Recent Advances in Core Needle Biopsy for Thyroid Nodules
Chan Kwon Jung, Jung Hwan Baek
Endocrinol Metab. 2017;32(4):407-412. doi: 10.3803/EnM.2017.32.4.407.Does Radiofrequency Ablation Induce Neoplastic Changes in Benign Thyroid Nodules: A Preliminary Study
Su Min Ha, Jun Young Shin, Jung Hwan Baek, Dong Eun Song, Sae Rom Chung, Young Jun Choi, Jeong Hyun Lee
Endocrinol Metab. 2019;34(2):169-178. doi: 10.3803/EnM.2019.34.2.169.RE: Thyroid Core Needle Biopsy: The Strengths of Guidelines of the Korean Society of Thyroid Radiology
Anna Crescenzi, Pierpaolo Trimboli
Korean J Radiol. 2017;18(5):867-869. doi: 10.3348/kjr.2017.18.5.867.Impact of Nodule Size on Malignancy Risk Differs according to the Ultrasonography Pattern of Thyroid Nodules
Min Ji Hong, Dong Gyu Na, Jung Hwan Baek, Jin Yong Sung, Ji-Hoon Kim
Korean J Radiol. 2018;19(3):534-541. doi: 10.3348/kjr.2018.19.3.534.Ultrasonographic Echogenicity and Histopathologic Correlation of Thyroid Nodules in Core Needle Biopsy Specimens
Ji-hoon Kim, Dong Gyu Na, Hunkyung Lee
Korean J Radiol. 2018;19(4):673-681. doi: 10.3348/kjr.2018.19.4.673.Evaluation of Modified Core-Needle Biopsy in the Diagnosis of Thyroid Nodules
Soomin Ahn, Sejin Jung, Ji-Ye Kim, Jung Hee Shin, Soo Yeon Hahn, Young Lyun Oh
Korean J Radiol. 2018;19(4):656-664. doi: 10.3348/kjr.2018.19.4.656.The Role of Core Needle Biopsy for the Evaluation of Thyroid Nodules with Suspicious Ultrasound Features
Sae Rom Chung, Jung Hwan Baek, Young Jun Choi, Tae-Yon Sung, Dong Eun Song, Tae Yong Kim, Jeong Hyun Lee
Korean J Radiol. 2019;20(1):158-165. doi: 10.3348/kjr.2018.0101.Core-Needle Biopsy Does Not Show Superior Diagnostic Performance to Fine-Needle Aspiration for Diagnosing Thyroid Nodules
Ilah Shin, Eun-Kyung Kim, Hee Jung Moon, Jung Hyun Yoon, Vivian Youngjean Park, Si Eun Lee, Hye Sun Lee, Jin Young Kwak
Yonsei Med J. 2020;61(2):161-168. doi: 10.3349/ymj.2020.61.2.161.The History of Korean Thyroid Pathology
Soon Won Hong, Chan Kwon Jung
Int J Thyroidol. 2018;11(1):15-20. doi: 10.11106/ijt.2018.11.1.15.Thyroid Fine-Needle Aspiration Cytology Practice in Korea
Yoon Jin Cha, Ju Yeon Pyo, SoonWon Hong, Jae Yeon Seok, Kyung-Ju Kim, Jee-Young Han, Jeong Mo Bae, Hyeong Ju Kwon, Yeejeong Kim, Kyueng-Whan Min, Soonae Oak, Sunhee Chang
J Pathol Transl Med. 2017;51(6):521-527. doi: 10.4132/jptm.2017.09.26.Contribution of cytologic examination to diagnosis of poorly differentiated thyroid carcinoma
Na Rae Kim, Jae Yeon Seok, Yoo Seung Chung, Joon Hyop Lee, Dong Hae Chung
J Pathol Transl Med. 2020;54(2):171-178. doi: 10.4132/jptm.2019.12.03.2019 Practice guidelines for thyroid core needle biopsy: a report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association
Chan Kwon Jung, Jung Hwan Baek, Dong Gyu Na, Young Lyun Oh, Ka Hee Yi, Ho-Cheol Kang
J Pathol Transl Med. 2020;54(1):64-86. doi: 10.4132/jptm.2019.12.04.Role of Immunohistochemistry in Fine Needle Aspiration and Core Needle Biopsy of Thyroid Nodules
Seulki Song, Hyojin Kim, Soon-Hyun Ahn
Clin Exp Otorhinolaryngol. 2019;12(2):224-230. doi: 10.21053/ceo.2018.01011.Usage and Diagnostic Yield of Fine-Needle Aspiration Cytology and Core Needle Biopsy in Thyroid Nodules: A Systematic Review and Meta-Analysis of Literature Published by Korean Authors
Soon-Hyun Ahn
Clin Exp Otorhinolaryngol. 2021;14(1):116-130. doi: 10.21053/ceo.2020.00199.Diagnostic Performance of Thyroid Core Needle Biopsy Using the Revised Reporting System: Comparison with Fine Needle Aspiration Cytology
Kwangsoon Kim, Ja Seong Bae, Jeong Soo Kim, So Lyung Jung, Chan Kwon Jung
Endocrinol Metab. 2022;37(1):159-169. doi: 10.3803/EnM.2021.1299.
Reference
-
1. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–214.
Article2. Ali SZ. Thyroid cytopathology: Bethesda and beyond. Acta Cytol. 2011; 55:4–12.
Article3. Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference. The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009; 132:658–65.
Article4. Lee SH, Kim MH, Bae JS, Lim DJ, Jung SL, Jung CK. Clinical outcomes in patients with non-diagnostic thyroid fine needle aspiration cytology: usefulness of the thyroid core needle biopsy. Ann Surg Oncol. 2014; 21:1870–7.
Article5. Hahn SY, Shin JH, Han BK, Ko EY, Ko ES. Ultrasonography-guided core needle biopsy for the thyroid nodule: does the procedure hold any benefit for the diagnosis when fine-needle aspiration cytology analysis shows inconclusive results? Br J Radiol. 2013; 86:20130007.
Article6. Trimboli P, Crescenzi A. Thyroid core needle biopsy: taking stock of the situation. Endocrine. 2015; 48:779–85.
Article7. Sung JY, Na DG, Kim KS, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol. 2012; 22:1564–72.
Article8. Na DG, Kim JH, Sung JY, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012; 22:468–75.
Article9. Baek JH, Na DG, Lee JH, et al. Core needle biopsy of thyroid nodules: consensus statement and recommendations. J Korean Soc Ultrasound Med. 2013; 32:95–102.10. Ha EJ, Baek JH, Lee JH, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol. 2014; 24:1403–9.
Article11. Yeon JS, Baek JH, Lim HK, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013; 268:274–80.
Article12. Trimboli P, Nasrollah N, Guidobaldi L, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. 2014; 12:61.
Article13. Nasrollah N, Trimboli P, Rossi F, et al. Patient’s comfort with and tolerability of thyroid core needle biopsy. Endocrine. 2014; 45:79–83.
Article14. Baloch ZW, LiVolsi VA. Our approach to follicular-patterned lesions of the thyroid. J Clin Pathol. 2007; 60:244–50.
Article15. Min HS, Kim JH, Ryoo I, Jung SL, Jung CK. The role of core needle biopsy in the preoperative diagnosis of follicular neoplasm of the thyroid. APMIS. 2014; 122:993–1000.
Article16. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007; 111:508–16.
Article17. Bae JS, Choi SK, Jeon S, et al. Impact of NRAS mutations on the diagnosis of follicular neoplasm of the thyroid. Int J Endocrinol. 2014; 2014:289834.
Article18. Ustun B, Chhieng D, Van Dyke A, et al. Risk stratification in follicular neoplasm: a cytological assessment using the modified Bethesda classification. Cancer Cytopathol. 2014; 122:536–45.
Article19. Sillery JC, Reading CC, Charboneau JW, Henrichsen TL, Hay ID, Mandrekar JN. Thyroid follicular carcinoma: sonographic features of 50 cases. AJR Am J Roentgenol. 2010; 194:44–54.
Article20. Reading CC, Charboneau JW, Hay ID, Sebo TJ. Sonography of thyroid nodules: a “classic pattern” diagnostic approach. Ultrasound Q. 2005; 21:157–65.21. Crescenzi A, Guidobaldi L, Nasrollah N, et al. Immunohistochemistry for BRAF(V600E) antibody VE1 performed in core needle biopsy samples identifies mutated papillary thyroid cancers. Horm Metab Res. 2014; 46:370–4.
Article22. Alshenawy HA. Utility of immunohistochemical markers in diagnosis of follicular cell derived thyroid lesions. Pathol Oncol Res. 2014; 20:819–28.
Article23. El Demellawy D, Nasr AL, Babay S, Alowami S. Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathol Res Pract. 2009; 205:303–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathology Reporting of Thyroid Core Needle Biopsy: A Proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group
- Surgical Management of Bleeding from the Superior Thyroid Artery after Core Needle Biopsy
- Recent Advances in Core Needle Biopsy for Thyroid Nodules
- RE: Thyroid Core Needle Biopsy: The Strengths of Guidelines of the Korean Society of Thyroid Radiology
- Thyroid Nodules with Nondiagnostic FNA Results: Role of Core Needle Biopsy