Korean J Physiol Pharmacol.
2012 Aug;16(4):265-271. 10.4196/kjpp.2012.16.4.265.
Increased HoxB4 Inhibits Apoptotic Cell Death in Pro-B Cells
- Affiliations
-
- 1Department of Biomedical Science, College of Life Science, CHA University, Seongnam 463-712, Korea.
- 2Department of Physiology and Medical Science, School of Medicine, Konkuk University, Chungju 380-701, Korea.
- 3Transplantation Research Center, Samsung Biomedical Research Institute, Seoul 135-710, Korea.
- 4Department of Pharmacology, Institute of Oral Biology, School of Dentistry, Kyung Hee University, Seoul 130-701, Korea.
- 5Laboratory of Animal Research, Asan Institute for Life Sciences, Asan Medical Center, Seoul 138-736, Korea.
- 6Department of Animal Biotechnology, Konkuk University, Seoul 143-701, Korea.
- KMID: 2011161
- DOI: http://doi.org/10.4196/kjpp.2012.16.4.265
Abstract
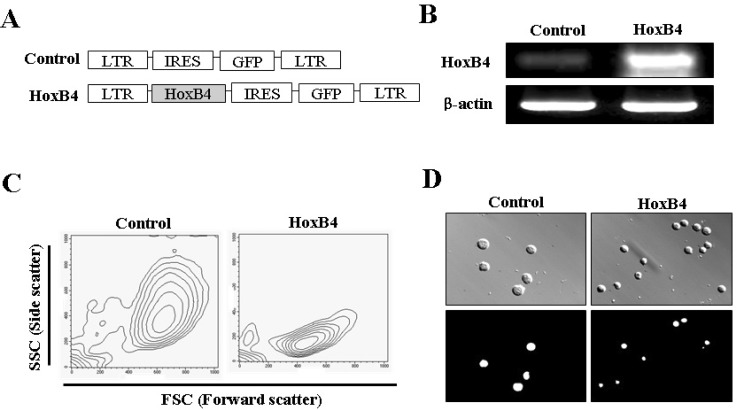
- HoxB4, a homeodomain-containing transcription factor, is involved in the expansion of hematopoietic stem cells and progenitor cells in vivo and in vitro, and plays a key role in regulating the balance between hematopoietic stem cell renewal and cell differentiation. However, the biological activity of HoxB4 in other cells has not been reported. In this study, we investigated the effect of overexpressed HoxB4 on cell survival under various conditions that induce death, using the Ba/F3 cell line. Analysis of phenotypical characteristics showed that HoxB4 overexpression in Ba/F3 cells reduced cell size, death, and proliferation rate. Moreover, the progression from early to late apoptotic stages was inhibited in Ba/F3 cells subjected to HoxB4 overexpression under removal of interleukin-3-mediated signal, leading to the induction of cell cycle arrest at the G2/M phase and attenuated cell death by Fas protein stimulation in vitro. Furthermore, apoptotic cell death induced by doxorubicin-treated G2/M phase cell-cycle arrest also decreased with HoxB4 overexpression in Ba/F3 cells. From these data, we suggest that HoxB4 may play an important role in the regulation of pro-B cell survival under various apoptotic death environments.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Ectopic Gene Expression Methods in Rat Neural Stem Cells
Woosuk Kim, Ji Hyeon Kim, Sun-Young Kong, Min-Hye Park, Uy Dong Sohn, Hyun-Jung Kim
Korean J Physiol Pharmacol. 2013;17(1):23-30. doi: 10.4196/kjpp.2013.17.1.23.
Reference
-
1. Deneault E, Cellot S, Faubert A, Laverdure JP, Fréchette M, Chagraoui J, Mayotte N, Sauvageau M, Ting SB, Sauvageau G. A functional screen to identify novel effectors of hematopoietic stem cell activity. Cell. 2009; 137:369–379. PMID: 19379700.
Article2. Opferman JT. Life and death during hematopoietic differentiation. Curr Opin Immunol. 2007; 19:497–502. PMID: 17662585.
Article3. Chen CZ, Lodish HF. MicroRNAs as regulators of mammalian hematopoiesis. Semin Immunol. 2005; 17:155–165. PMID: 15737576.
Article4. Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005; 6:314–322. PMID: 15665828.
Article5. Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008; 111:492–503. PMID: 17914027.
Article6. Sauvageau G, Lansdorp PM, Eaves CJ, Hogge DE, Dragowska WH, Reid DS, Largman C, Lawrence HJ, Humphries RK. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci USA. 1994; 91:12223–12227. PMID: 7527557.
Article7. Daley GQ, Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci USA. 1988; 85:9312–9316. PMID: 3143116.
Article8. Ajjappala BS, Kim YS, Kim MS, Lee MY, Lee KY, Ki HY, Cha DH, Baek KH. 14-3-3 gamma is stimulated by IL-3 and promotes cell proliferation. J Immunol. 2009; 182:1050–1060. PMID: 19124748.9. Magli MC, Barba P, Celetti A, De Vita G, Cillo C, Boncinelli E. Coordinate regulation of HOX genes in human hematopoietic cells. Proc Natl Acad Sci USA. 1991; 88:6348–6352. PMID: 1712489.
Article11. Martinez P, Amemiya CT. Genomics of the HOX gene cluster. Comp Biochem Physiol B Biochem Mol Biol. 2002; 133:571–580. PMID: 12470820.
Article12. Svingen T, Tonissen KF. Hox transcription factors and their elusive mammalian gene targets. Heredity (Edinb). 2006; 97:88–96. PMID: 16721389.
Article13. Buske C, Feuring-Buske M, Abramovich C, Spiekermann K, Eaves CJ, Coulombel L, Sauvageau G, Hogge DE, Humphries RK. Deregulated expression of HOXB4 enhances the primitive growth activity of human hematopoietic cells. Blood. 2002; 100:862–868. PMID: 12130496.
Article14. Will E, Speidel D, Wang Z, Ghiaur G, Rimek A, Schiedlmeier B, Williams DA, Baum C, Ostertag W, Klump H. HOXB4 inhibits cell growth in a dose-dependent manner and sensitizes cells towards extrinsic cues. Cell Cycle. 2006; 5:14–22. PMID: 16357528.
Article15. Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002; 109:39–45. PMID: 11955445.
Article16. Milsom MD, Schiedlmeier B, Bailey J, Kim MO, Li D, Jansen M, Ali AM, Kirby M, Baum C, Fairbairn LJ, Williams DA. Ectopic HOXB4 overcomes the inhibitory effect of tumor necrosis factor-{alpha} on Fanconi anemia hematopoietic stem and progenitor cells. Blood. 2009; 113:5111–5120. PMID: 19270262.17. Schiedlmeier B, Santos AC, Ribeiro A, Moncaut N, Lesinski D, Auer H, Kornacker K, Ostertag W, Baum C, Mallo M, Klump H. HOXB4's road map to stem cell expansion. Proc Natl Acad Sci USA. 2007; 104:16952–16957. PMID: 17940039.
Article18. Gamen S, Anel A, Lasierra P, Alava MA, Martinez-Lorenzo MJ, Piñeiro A, Naval J. Doxorubicin-induced apoptosis in human T-cell leukemia is mediated by caspase-3 activation in a Fas-independent way. FEBS Lett. 1997; 417:360–364. PMID: 9409752.
Article19. Kim HS, Lee YS, Kim DK. Doxorubicin exerts cytotoxic effects through cell cycle arrest and Fas-mediated cell death. Pharmacology. 2009; 84:300–309. PMID: 19829019.
Article20. Haddad R, Pflumio F, Vigon I, Visentin G, Auvray C, Fichelson S, Amsellem S. The HOXB4 homeoprotein differentially promotes ex vivo expansion of early human lymphoid progenitors. Stem Cells. 2008; 26:312–322. PMID: 17962697.
Article21. Scatizzi JC, Mavers M, Hutcheson J, Young B, Shi B, Pope RM, Ruderman EM, Samways DS, Corbett JA, Egan TM, Perlman H. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol. 2009; 39:820–825. PMID: 19189309.22. See WA, Zhang G, Chen F, Cao Y. p21 Expression by human urothelial carcinoma cells modulates the phenotypic response to BCG. Urol Oncol. 2010; 28:526–533. PMID: 19450997.
Article23. Kim CH, Yoo YM. Melatonin induces apoptotic cell death via p53 in LNCaP cells. Korean J Physiol Pharmacol. 2010; 14:365–369. PMID: 21311676.
Article24. Chung JS, Lee SB, Park SH, Kang ST, Na AR, Chang TS, Kim HJ, Yoo YD. Mitochondrial reactive oxygen species originating from Romo1 exert an important role in normal cell cycle progression by regulating p27(Kip1) expression. Free Radic Res. 2009; 43:729–737. PMID: 19513905.25. Kim KC, Lee C. Curcumin induces downregulation of E2F4 expression and apoptotic cell death in HCT116 human colon cancer cells; Involvement of reactive oxygen species. Korean J Physiol Pharmacol. 2010; 14:391–397. PMID: 21311680.
Article26. Aaltonen K, Amini RM, Landberg G, Eerola H, Aittomäki K, Heikkilä P, Nevanlinna H, Blomqvist C. Cyclin D1 expression is associated with poor prognostic features in estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2009; 113:75–82. PMID: 18240019.
Article27. Krosl J, Sauvageau G. AP-1 complex is effector of Hox-induced cellular proliferation and transformation. Oncogene. 2000; 19:5134–5141. PMID: 11064450.
Article28. Zhu N, Shao Y, Xu L, Yu L, Sun L. Gadd45-alpha and Gadd45-gamma utilize p38 and JNK signaling pathways to induce cell cycle G2/M arrest in Hep-G2 hepatoma cells. Mol Biol Rep. 2009; 36:2075–2085. PMID: 19048389.29. Jin S, Tong T, Fan W, Fan F, Antinore MJ, Zhu X, Mazzacurati L, Li X, Petrik KL, Rajasekaran B, Wu M, Zhan Q. GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity. Oncogene. 2002; 21:8696–8704. PMID: 12483522.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ghrelin Inhibits Oligodendrocyte Cell Death by Attenuating Microglial Activation
- Running Title: Apoptotic Effect of Mycolactone in SCC15 Cells
- Effects of LED irradiation on the expression of apoptosis-related molecules in human SH-SY5Y neuroblastoma cells
- Elm tree bark extract inhibits HepG2 hepatic cancer cell growth via pro-apoptotic activity
- Protective Effects of Epigallocatechin-3-gallate against 1-methyl-4-phenylpyridinium-induced Dopaminergic Neuronal Cell Death