Renoprotective Effect of Gemigliptin, a Dipeptidyl Peptidase-4 Inhibitor, in Streptozotocin-Induced Type 1 Diabetic Mice
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea. kpark@knu.ac.kr
- 2Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea. mdkmk@dsmc.or.kr
- KMID: 2308629
- DOI: http://doi.org/10.4093/dmj.2016.40.3.211
Abstract
- BACKGROUND
Dipeptidyl peptidase-4 (DPP-4) inhibitors are widely used in the treatment of patients with type 2 diabetes and have proven protective effects on diabetic kidney disease (DKD). Whether DPP-4 inhibitors have renoprotective effects on insulin-deficient type 1 diabetes has not been comprehensively examined. The aim of this study was to determine whether gemigliptin, a new DPP-4 inhibitor, has renoprotective effects in streptozotocin (STZ)-induced type 1 diabetic mice.
METHODS
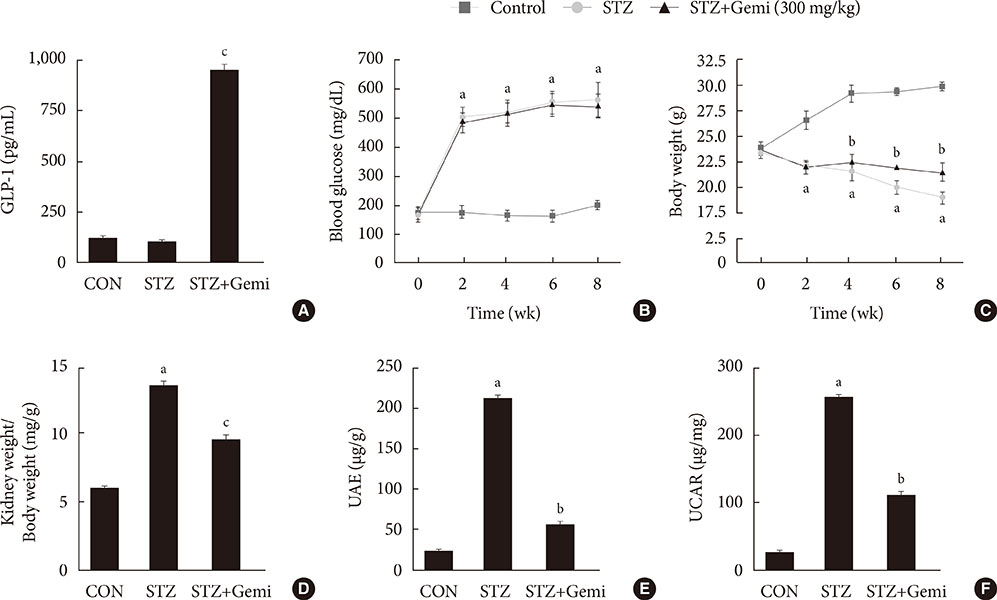
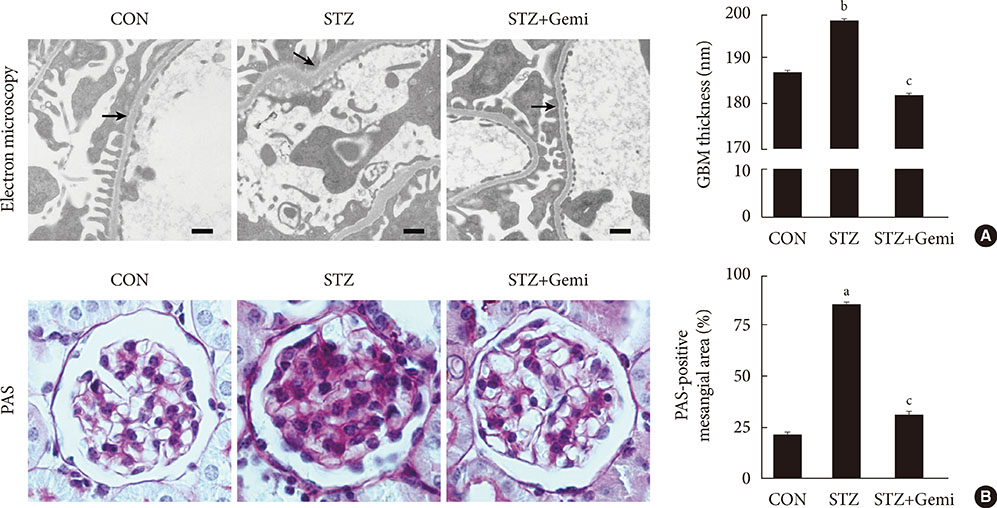
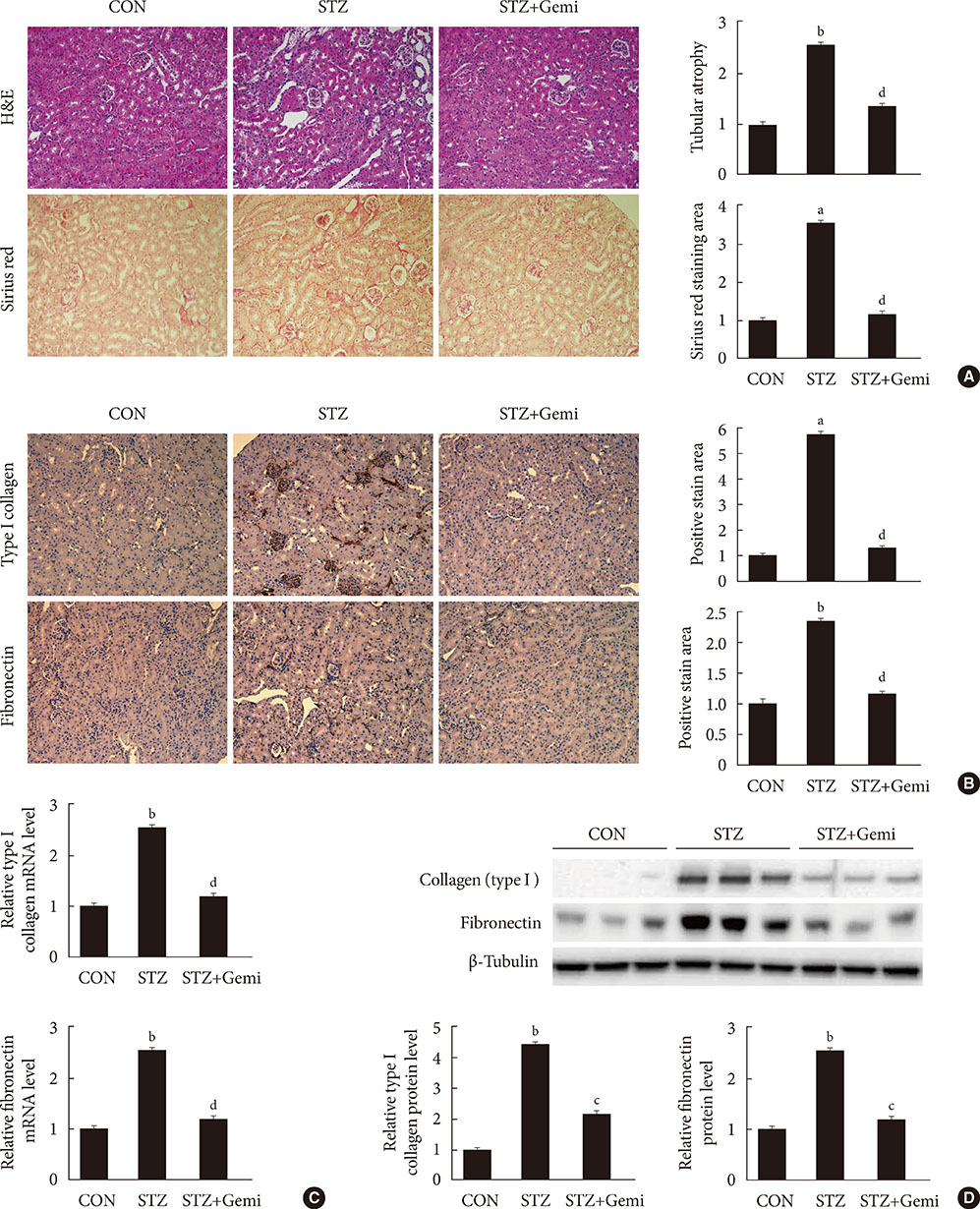
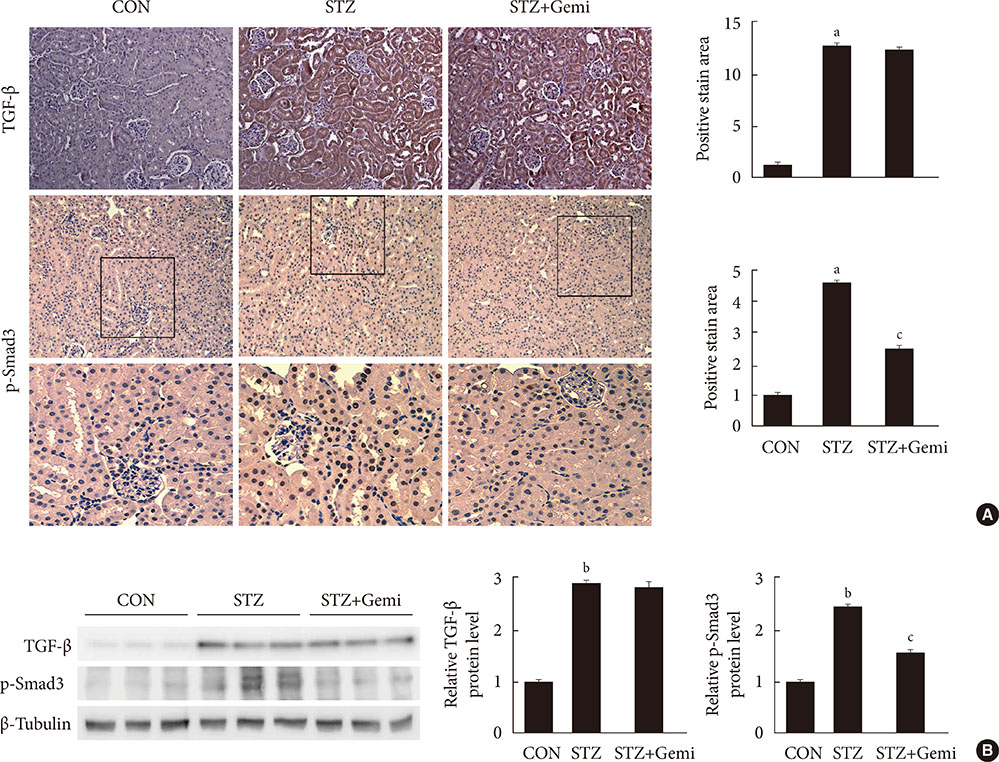
Diabetes was induced by intraperitoneal administration of a single dose of STZ. Mice with diabetes were treated without or with gemigliptin (300 mg/kg) for 8 weeks. Morphological changes of the glomerular basement membrane (GBM) were observed by electron microscopy and periodic-acid Schiff staining. In addition, we measured blood glucose and urinary albumin excretion and evaluated fibrotic markers using immunohistochemical staining, quantitative reverse transcription polymerase chain reaction analysis, and Western blot analysis.
RESULTS
Gemigliptin did not reduce the blood glucose levels of STZ-treated mice. In gemigliptin-treated mice with STZ, a significant reduction in urinary albumin excretion and GBM thickness was observed. Immunohistological examination revealed that gemigliptin attenuated renal fibrosis induced by STZ and decreased extracellular matrix protein levels, including those of type I collagen and fibronectin, and Smad3 phosphorylation. In cultured rat renal cells, gemigliptin inhibited transforming growth factor β-stimulated type I collagen and fibronectin mRNA and protein levels via down-regulation of Smad3 phosphorylation.
CONCLUSION
Our data demonstrate that gemigliptin has renoprotective effects on DKD, regardless of its glucose-lowering effect, suggesting that it could be used to prevent DKD, including in patients with type 1 diabetes.
MeSH Terms
-
Animals
Blood Glucose
Blotting, Western
Collagen Type I
Diabetes Mellitus, Type 1
Diabetic Nephropathies
Down-Regulation
Extracellular Matrix
Fibronectins
Fibrosis
Glomerular Basement Membrane
Humans
Mice*
Microscopy, Electron
Phosphorylation
Polymerase Chain Reaction
Rats
Reverse Transcription
RNA, Messenger
Streptozocin
Transforming Growth Factors
Blood Glucose
Collagen Type I
Fibronectins
RNA, Messenger
Streptozocin
Transforming Growth Factors
Figure
Cited by 4 articles
-
Evogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Attenuates Renal Fibrosis Caused by Unilateral Ureteral Obstruction in Mice
Mi-Jin Kim, Na-young Kim, Yun-A Jung, Seunghyeong Lee, Gwon-Soo Jung, Jung-Guk Kim, In-Kyu Lee, Sungwoo Lee, Yeon-Kyung Choi, Keun-Gyu Park
Diabetes Metab J. 2020;44(1):186-192. doi: 10.4093/dmj.2018.0271.Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
Jung Beom Seo, Yeon-Kyung Choi, Hye-In Woo, Yun-A Jung, Sungwoo Lee, Seunghyeong Lee, Mihyang Park, In-Kyu Lee, Gwon-Soo Jung, Keun-Gyu Park
Diabetes Metab J. 2019;43(6):830-839. doi: 10.4093/dmj.2018.0181.Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis
Jae Hyun Bae, Sunhee Kim, Eun-Gee Park, Sin Gon Kim, Seokyung Hahn, Nam Hoon Kim
Endocrinol Metab. 2019;34(1):80-92. doi: 10.3803/EnM.2019.34.1.80.Sodium butyrate has context-dependent actions on dipeptidyl peptidase-4 and other metabolic parameters
Eun-Sol Lee, Dong-Sung Lee, Prakash Raj Pandeya, Youn-Chul Kim, Dae-Gil Kang, Ho-Sub Lee, Byung-Chul Oh, Dae Ho Lee
Korean J Physiol Pharmacol. 2017;21(5):519-529. doi: 10.4196/kjpp.2017.21.5.519.
Reference
-
1. Packham DK, Alves TP, Dwyer JP, Atkins R, de Zeeuw D, Cooper M, Shahinfar S, Lewis JB, Lambers Heerspink HJ. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am J Kidney Dis. 2012; 59:75–83.2. Park CW. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J. 2014; 38:252–260.3. Muskiet MH, Smits MM, Morsink LM, Diamant M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014; 10:88–103.4. Lan HY, Chung AC. TGF-β/Smad signaling in kidney disease. Semin Nephrol. 2012; 32:236–243.5. Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, Han SW, Shin SJ, Bang BK, Breyer MD, Chang YS. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007; 18:1227–1238.6. Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011; 54:965–978.7. Hendarto H, Inoguchi T, Maeda Y, Ikeda N, Zheng J, Takei R, Yokomizo H, Hirata E, Sonoda N, Takayanagi R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. 2012; 61:1422–1434.8. Mentlein R. Dipeptidyl-peptidase IV (CD26): role in the inactivation of regulatory peptides. Regul Pept. 1999; 85:9–24.9. Weber AE. Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem. 2004; 47:4135–4141.10. Deacon CF, Holst JJ. Dipeptidyl peptidase IV inhibition as an approach to the treatment and prevention of type 2 diabetes: a historical perspective. Biochem Biophys Res Commun. 2002; 294:1–4.11. Cernea S, Raz I. Therapy in the early stage: incretins. Diabetes Care. 2011; 34:Suppl 2. S264–S271.12. Chrysant SG, Chrysant GS. Clinical implications of cardiovascular preventing pleiotropic effects of dipeptidyl peptidase-4 inhibitors. Am J Cardiol. 2012; 109:1681–1685.13. Min HS, Kim JE, Lee MH, Song HK, Kang YS, Lee MJ, Lee JE, Kim HW, Cha JJ, Chung YY, Hyun YY, Han JY, Cha DR. Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction. Lab Invest. 2014; 94:598–607.14. Kodera R, Shikata K, Takatsuka T, Oda K, Miyamoto S, Kajitani N, Hirota D, Ono T, Usui HK, Makino H. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun. 2014; 443:828–833.15. Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, Shao YM, Park TS. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2012; 340:248–255.16. Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014; 63:2120–2131.17. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369:1317–1326.18. White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013; 369:1327–1335.19. Mori H, Okada Y, Arao T, Tanaka Y. Sitagliptin improves albuminuria in patients with type 2 diabetes mellitus. J Diabetes Investig. 2014; 5:313–319.20. Fujita H, Taniai H, Murayama H, Ohshiro H, Hayashi H, Sato S, Kikuchi N, Komatsu T, Komatsu K, Komatsu K, Narita T, Yamada Y. DPP-4 inhibition with alogliptin on top of angiotensin II type 1 receptor blockade ameliorates albuminuria via up-regulation of SDF-1α in type 2 diabetic patients with incipient nephropathy. Endocr J. 2014; 61:159–166.21. Kim SH, Lee SH, Yim HJ. Gemigliptin, a novel dipeptidyl peptidase 4 inhibitor: first new anti-diabetic drug in the history of Korean pharmaceutical industry. Arch Pharm Res. 2013; 36:1185–1188.22. Kim N, Patrick L, Mair S, Stevens L, Ford G, Birks V, Lee SH. Absorption, metabolism and excretion of [14C]gemigliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Xenobiotica. 2014; 44:522–530.23. Hwang HJ, Chung HS, Jung TW, Ryu JY, Hong HC, Seo JA, Kim SG, Kim NH, Choi KM, Choi DS, Baik SH, Yoo HJ. The dipeptidyl peptidase-IV inhibitor inhibits the expression of vascular adhesion molecules and inflammatory cytokines in HUVECs via Akt- and AMPK-dependent mechanisms. Mol Cell Endocrinol. 2015; 405:25–34.24. Jung GS, Kim MK, Jung YA, Kim HS, Park IS, Min BH, Lee KU, Kim JG, Park KG, Lee IK. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol. 2012; 23:73–85.25. Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004; 15:Suppl 1. S55–S57.26. Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol. 2010; 21:1477–1487.27. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004; 18:816–827.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Renoprotective Effect of Gemigliptin, a Dipeptidyl Peptidase-4 Inhibitor, in Streptozotocin-Induced Type 1 Diabetic Mice
- Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus
- Potentiation of endothelium-dependent vasorelaxation of mesenteric arteries from spontaneously hypertensive rats by gemigliptin, a dipeptidyl peptidase-4 inhibitor class of anti-diabetic drug