Diabetes Metab J.
2012 Aug;36(4):255-261. 10.4093/dmj.2012.36.4.255.
The Role of Oxidative Stress in the Pathogenesis of Diabetic Vascular Complications
- Affiliations
-
- 1Department of Medicine and Regulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan. toyoshi@intmed3.med.kyushu-u.ac.jp
- 2Innovation Center for Medical Redox Navigation, Kyushu University, Fukuoka, Japan.
- KMID: 2174415
- DOI: http://doi.org/10.4093/dmj.2012.36.4.255
Abstract
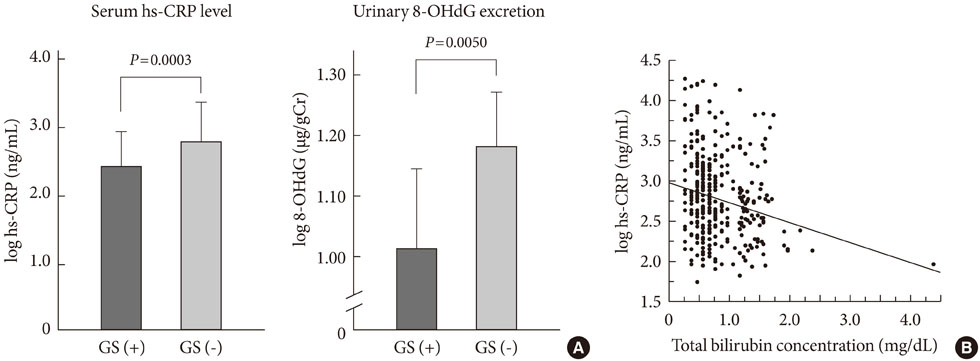
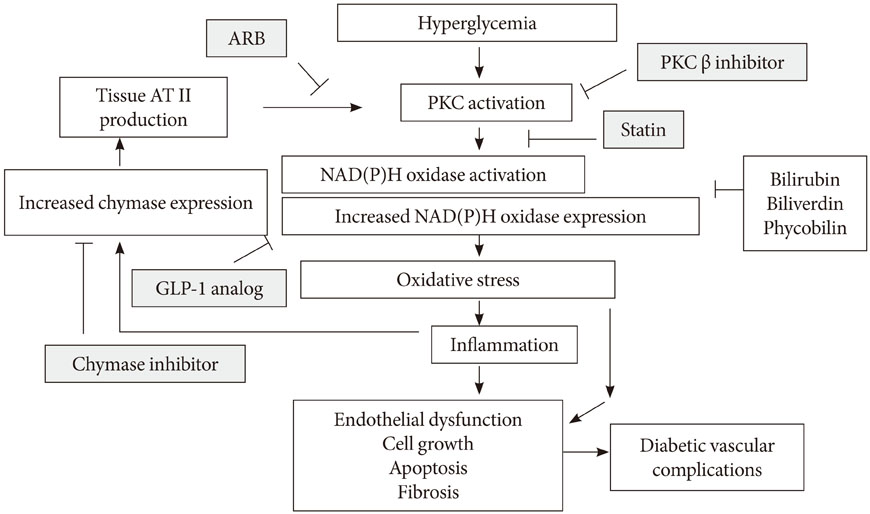
- Oxidative stress has been paid increasing attention to as an important causative factor for diabetic vascular complications. Among possible various sources, accumulating evidence has indicated that NAD(P)H oxidase may be the most important source for reactive oxygen species production in diabetic vascular tissues. The mechanisms underlying activation and up-regulation of NAD(P)H oxidase has been supposed to be mediated by high glucose-induced protein kinase C (PKC) activation. In this review article, activation of local renin-angiotensin II system induced by chymase activation is also shown to amplify such a PKC-dependent activation of NAD(P)H oxidase. Additionally, human evidence showing the beneficial effect of antioxidants on diabetic vascular complications. Bilirubin has been recognized as a strong endogenous antioxidant. Here markedly lower prevalence of vascular complications is shown in diabetic patients with Gilbert syndrome, a congenital hyperbilirubinemia, as well as reduced markers of oxidative stress and inflammation. Lastly, statin, angiotensin II receptor blocker, chymase inhibitor, bilirubin and biliverdin, PKC beta isoform inhibitor, and glucagon-like peptide-1 analog, are shown to serve as antioxidants and have some beneficial effect on diabetic vascular complications, via inhibiting PKC-NAD(P)H oxidase activation, supporting the notion that this mechanism may be an effective therapeutic target for preventing diabetic vascular complications.
MeSH Terms
-
Angiotensin II
Antioxidants
Bilirubin
Biliverdine
Chymases
Diabetic Angiopathies
Gilbert Disease
Glucagon-Like Peptide 1
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Hyperbilirubinemia
Inflammation
NADPH Oxidase
Oxidative Stress
Oxidoreductases
Prevalence
Protein Kinase C
Reactive Oxygen Species
Receptors, Angiotensin
Up-Regulation
Angiotensin II
Antioxidants
Bilirubin
Biliverdine
Chymases
Glucagon-Like Peptide 1
Hydroxymethylglutaryl-CoA Reductase Inhibitors
NADPH Oxidase
Oxidoreductases
Protein Kinase C
Reactive Oxygen Species
Receptors, Angiotensin
Figure
Cited by 2 articles
-
Comparison of antioxidant, α-glucosidase inhibition and anti-inflammatory activities of the leaf and root extracts of Smilax china L.
Kyoung Kon Kim, Yun Hwan Kang, Dae Jung Kim, Tae Woo Kim, Myeon Choe
J Nutr Health. 2013;46(4):315-323. doi: 10.4163/jnh.2013.46.4.315.Pathophysiologic Mechanisms and Potential Biomarkers in Diabetic Kidney Disease
Chan-Young Jung, Tae-Hyun Yoo
Diabetes Metab J. 2022;46(2):181-197. doi: 10.4093/dmj.2021.0329.
Reference
-
1. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991. 40:405–412.2. Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988. 5:113–124.3. Ha H, Kim C, Son Y, Chung MH, Kim KH. DNA damage in the kidneys of diabetic rats exhibiting microalbuminuria. Free Radic Biol Med. 1994. 16:271–274.4. Kakimoto M, Inoguchi T, Sonta T, Yu HY, Imamura M, Etoh T, Hashimoto T, Nawata H. Accumulation of 8-hydroxy-2'-deoxyguanosine and mitochondrial DNA deletion in kidney of diabetic rats. Diabetes. 2002. 51:1588–1595.5. Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med. 2004. 37:115–123.6. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000. 404:787–790.7. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C: dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1945.8. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001. 88:E14–E22.9. Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW, Kim CD. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes. 2002. 51:522–527.10. Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002. 105:1656–1662.11. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003. 14:8 Suppl 3. S227–S232.12. Etoh T, Inoguchi T, Kakimoto M, Sonoda N, Kobayashi K, Kuroda J, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia. 2003. 46:1428–1437.13. Tsubouchi H, Inoguchi T, Sonta T, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Statin attenuates high glucose-induced and diabetes-induced oxidative stress in vitro and in vivo evaluated by electron spin resonance measurement. Free Radic Biol Med. 2005. 39:444–452.14. Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M. Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003. 52:2603–2614.15. Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006. 55:3112–3120.16. Sonta T, Inoguchi T, Matsumoto S, Yasukawa K, Inuo M, Tsubouchi H, Sonoda N, Kobayashi K, Utsumi H, Nawata H. In vivo imaging of oxidative stress in the kidney of diabetic mice and its normalization by angiotensin II type 1 receptor blocker. Biochem Biophys Res Commun. 2005. 330:415–422.17. Huang XR, Chen WY, Truong LD, Lan HY. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol. 2003. 14:1738–1747.18. Maeda Y, Inoguchi T, Takei R, Sawada F, Sasaki S, Fujii M, Kobayashi K, Urata H, Nishiyama A, Takayanagi R. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am J Physiol Renal Physiol. 2010. 299:F1328–F1338.19. Maeda Y, Inoguchi T, Takei R, Hendarto H, Ide M, Inoue T, Kobayashi K, Urata H, Nishiyama A, Takayanagi R. Chymase inhibition prevents myocardial fibrosis through the attenuation of NOX4-associated oxidative stress in diabetic hamsters. J Diabetes Investig. Epub 2012 Feb 21. DOI: http://dx.doi.org/10.1111/j.2040-1124.2012.00202.x.20. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987. 235:1043–1046.21. Lanone S, Bloc S, Foresti R, Almolki A, Taille C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, El-Benna J, Motterlini R, Boczkowski J. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005. 19:1890–1892.22. Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA. 2007. 298:1398–1400.23. Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, Hasegawa G, Nakamura N. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int. 2008. 74:1197–1201.24. Yasuda M, Kiyohara Y, Wang JJ, Arakawa S, Yonemoto K, Doi Y, Ninomiya T, Ishibashi T. High serum bilirubin levels and diabetic retinopathy: the Hisayama Study. Ophthalmology. 2011. 118:1423–1428.25. Ohnaka K, Kono S, Inoguchi T, Yin G, Morita M, Adachi M, Kawate H, Takayanagi R. Inverse associations of serum bilirubin with high sensitivity C-reactive protein, glycated hemoglobin, and prevalence of type 2 diabetes in middle-aged and elderly Japanese men and women. Diabetes Res Clin Pract. 2010. 88:103–110.26. Han SS, Na KY, Chae DW, Kim YS, Kim S, Chin HJ. High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. Tohoku J Exp Med. 2010. 221:133–140.27. Choi SH, Yun KE, Choi HJ. Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutr Metab Cardiovasc Dis. Epub 2011 Jun 22. DOI: http://dx.doi.org/10.1016/j.numecd.2011.03.001.28. Inoguchi T, Nawata H. NAD(P)H oxidase activation: a potential target mechanism for diabetic vascular complications, progressive beta-cell dysfunction and metabolic syndrome. Curr Drug Targets. 2005. 6:495–501.29. Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int. 2007. 72:473–480.30. Kimura S, Inoguchi T, Yokomizo H, Maeda Y, Sonoda N, Takayanagi R. Randomized comparison of pitavastatin and pravastatin treatment on the reduction of urinary albumin in patients with type 2 diabetic nephropathy. Diabetes Obes Metab. 2012. 14:666–669.31. Nakayama M, Inoguchi T, Sonta T, Maeda Y, Sasaki S, Sawada F, Tsubouchi H, Sonoda N, Kobayashi K, Sumimoto H, Nawata H. Increased expression of NAD(P)H oxidase in islets of animal models of type 2 diabetes and its improvement by an AT1 receptor antagonist. Biochem Biophys Res Commun. 2005. 332:927–933.32. Fujii M, Inoguchi T, Sasaki S, Maeda Y, Zheng J, Kobayashi K, Takayanagi R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int. 2010. 78:905–919.33. Ikeda N, Inoguchi T, Sonoda N, Fujii M, Takei R, Hirata E, Yokomizo H, Zheng J, Maeda Y, Kobayashi K, Takayanagi R. Biliverdin protects against the deterioration of glucose tolerance in db/db mice. Diabetologia. 2011. 54:2183–2191.34. Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992. 89:11059–11063.35. Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996. 272:728–731.36. He Z, King GL. Can protein kinase C beta-selective inhibitor, ruboxistaurin, stop vascular complications in diabetic patients? Diabetes Care. 2005. 28:2803–2805.37. Hendarto H, Inoguchi T, Maeda Y, Ikeda N, Zheng J, Takei R, Yokomizo H, Hirata E, Sonoda N, Takayanagi R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism. Epub 2012 May 1. DOI: http://dx.doi.org/10.1016/j.metabol.2012.03.002.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reactive Oxygen and Nitrogen Species in Pathogenesis of Vascular Complications of Diabetes
- The Role of Oxidative Stress in the Pathogenesis of Diabetic Vascular Complications
- Mitochondrial oxidative stress regulatory approaches for diabetic encephalopathy
- Mechanism of Developing Diabetic Vascular Complication by Oxidative Stress
- Effects of S-allylcysteine on Oxidative Stress in Streptozotocin-Induced Diabetic Rats