Korean J Urol.
2006 Apr;47(4):347-352. 10.4111/kju.2006.47.4.347.
The Clinical Significance of the Expression of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 in Renal Cell Carcinoma
- Affiliations
-
- 1Departments of Urology, Yeungnam University College of Medicine, Daegu, Korea.
- 2Departments of Biochemistry and Molecular Biology, Yeungnam University College of Medicine, Daegu, Korea.
- KMID: 1997140
- DOI: http://doi.org/10.4111/kju.2006.47.4.347
Abstract
- PURPOSE
Matrix metalloproteinases (MMPs) are endogenous peptidases that are capable of degrading various components of the basement membranes. To evaluate the clinical significance of the expressions of MMPs in renal cell carcinomas (RCCs), the MMPs' expression in RCCs and non- neoplastic kidney tissues was examined to evaluate the clinical significance of the expressions of MMPs in renal cell carcinomas (RCCs).
MATERIALS AND METHODS
Twenty-two patients with RCCs (the RCC group), and eleven patients with non-neoplastic kidneys (the control group), were enrolled in this study between November 2002 and November 2003. The MMP-2 and MMP-9 activities were estimated using gelatin zymography, and they were quantified using a laser densitometer. The results were compared with the clinicopathological characteristics.
RESULTS
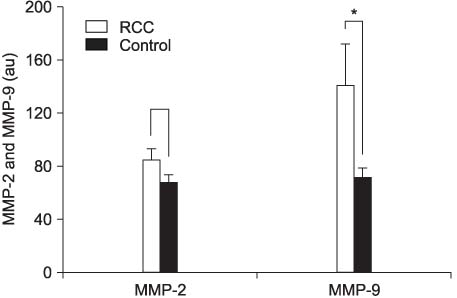
The expression of MMP-9 was significantly elevated in the RCC group compared with the control group (p<0.01). There was no difference in MMP-2 activity between the RCC group and the control group (p>0.05). The levels of MMP-9 expression in the RCC patients with a large tumor (>4cm) or vascular invasion were significantly higher than that in the patients without these clinical manifestations (p<0.01). There were also significant differences in the expression of MMP-9 among the T stages (p<0.01).
CONCLUSIONS
The present study shows a close relationship between the expression of MMP-9 and the tumor size and tumor stage in RCC. MMP-9 may be used as a prognostic marker and for the development of a novel treatment modality for RCC.
MeSH Terms
Figure
Reference
-
1. Linehan WM, Zbar B, Bates SE, Zelefsky MJ, Yang JC. Devita VT, Hellman S, Rosenberg SA, editors. Cancer of the kidney and ureter. Cancer: principles and practice of oncology. 2001. 6th ed. Philadelphia: Lippincott;1362–1395.2. Rafla S. Renal cell carcinoma. Natural history and results of treatment. Cancer. 1970. 25:26–40.3. Ficarra V, Righetti R, Pilloni S, D'amico A, Maffei N, Novella G, et al. Prognostic factors in patients with renal cell carcinoma: retrospective analysis of 675 cases. Eur Urol. 2002. 41:190–198.4. Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000. 36:1621–1630.5. Murray GI. Matrix metalloproteinases: a multifunctional group of molecules. J Pathol. 2001. 195:135–137.6. Tryggvason K, Hoyhtya M, Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993. 24:209–218.7. Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002. 21:2245–2252.8. Sobin LH, Wittekind CH. TNM classification of malignant tumours: UICC International Union Against Cancer. 2003. 6th ed. New York: Wiley-Liss;193.9. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982. 6:655–663.10. Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000. 163:408–417.11. Figlin RA. Renal cell carcinoma: management of advanced disease. J Urol. 1999. 161:381–386.12. Liotta LA. Tumor invasion and metastases-role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986. 46:1–7.13. Nakajima M, Welch DR, Belloni PN, Nicolson GL. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987. 47:4869–4876.14. Tryggvason K, Hoyhtya M, Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987. 907:191–217.15. Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988. 263:6579–6587.16. Nicolson GL. Metastatic tumor cell interactions with endothelium, basement membrane and tissue. Curr Opin Cell Biol. 1989. 1:1009–1019.17. Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994. 370:61–65.18. Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 1994. 218:325–329.19. Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinsases and metastasis. Cancer Chemother Pharmacol. 1999. 43:Suppl. S42–S51.20. Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, et al. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996. 24:316–322.21. Sato H, Kida Y, Mai M, Endo Y, Sasaki T, Tanaka J, et al. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene. 1992. 7:77–83.22. Rao JS, Steck PA, Mohanam S, Stetler-Stevenson WG, Liotta LA, Sawaya R. Elevated levels of M (r) 92,000 type IV collagenase in human brain tumors. Cancer Res. 1993. 53:Suppl 10. S2208–S2211.23. Kugler A, Hemmerlein B, Thelen P, Kallerhoff M, Radzun HJ, Ringert RH. Expression of metalloproteinase 2 and 9 and their inhibitors in renal cell carcinoma. J Urol. 1998. 160:1914–1918.24. Lein M, Jung K, Laube C, Hubner T, Winkelmann B, Stephan C, et al. Matrix-metalloproteinases and their inhibitors in plasma and tumor tissues of patients with renal cell carcinoma. Int J Cancer. 2000. 85:801–804.25. Walther MM, Kleiner DE, Lubensky IA, Pozzatti R, Nyguen T, Gnarra JR, et al. Progelatinase A mRNA expression in cell lines derived from tumors in patients with metastatic renal cell carcinoma correlates inversely with survival. Urology. 1997. 50:295–301.26. Zeng ZS, Huang Y, Cohen AM, Guillem JG. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9. J Clin Oncol. 1996. 14:3133–3140.27. Sier CF, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, et al. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br J Cancer. 1996. 74:413–417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Matrix Metalloproteinase-9 in Asthma
- Analysis of Matrix Metalloproteinase-9 Expression in Renal Cell Carcinoma
- EGCC inhibits tumor growth by inbibiting Matrix Metalloproteinase-9 induction in UM-SCC-1 cells
- The Effects of Hantaan Virus on Fibronectin and Matrix Metalloproteinase-3
- Expression of MMP-2, MT1-MMP, and TIMP-2 mRNA in Breast Carcinomas