Anat Cell Biol.
2014 Jun;47(2):111-116. 10.5115/acb.2014.47.2.111.
Change of peroxisome proliferator-activated receptor gamma expression pattern in the gerbil dentate gyrus after transient global cerebral ischemia
- Affiliations
-
- 1Department of Pharmacy, College of Pharmacy, Dankook University, Cheonan, Korea.
- 2Department of Neurobiology and Institute of Medical Sciences, Kangwon National University School of Medicine, Chuncheon, Korea. mhwon@kangwon.ac.kr
- KMID: 1882581
- DOI: http://doi.org/10.5115/acb.2014.47.2.111
Abstract
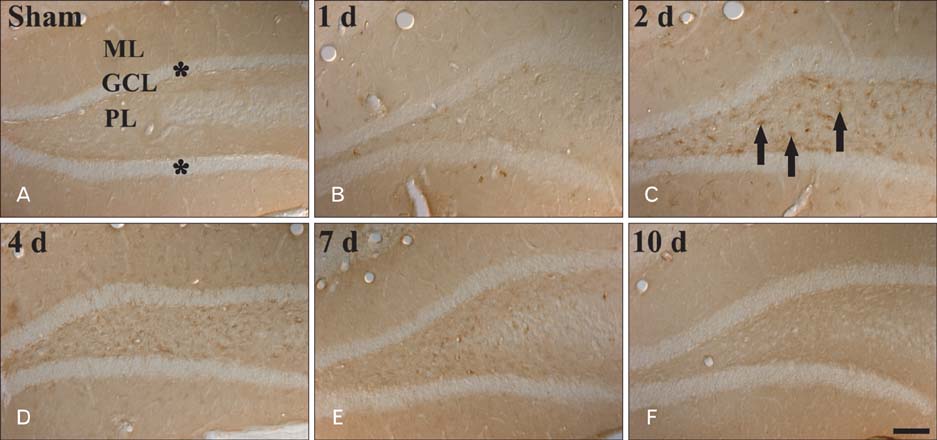
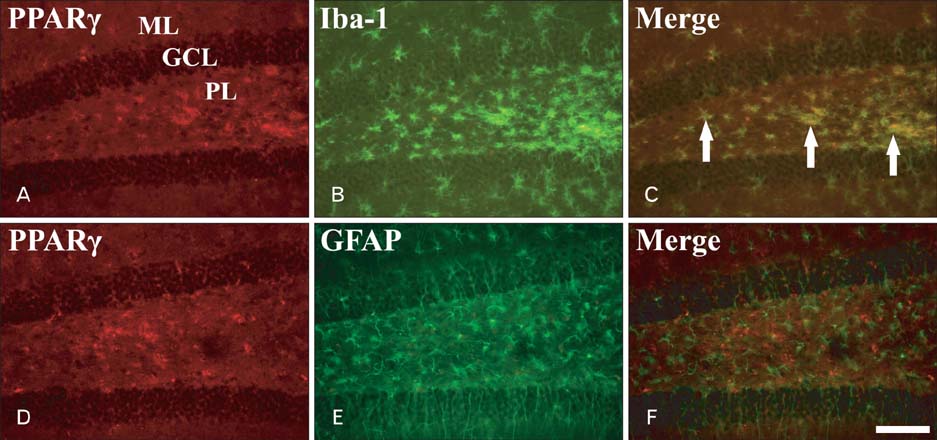
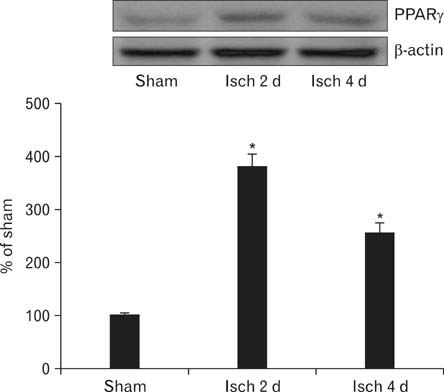
- Peroxisome proliferator-activated receptor gamma (PPARgamma) has various actions including the regulation of adipocyte differentiation, lipid metabolism and glucose homeostasis. In the present study, we examined the changes of PPARgamma immunoreactivity and protein levels in the gerbil dentate gyrus (DG) after transient global cerebral ischemia using immunohistochemistry and western blot analysis. PPARgamma immunoreactivity was gradually increased from 1 day after ischemia-reperfusion. PPARgamma immunoreactivity, in accordance with protein level, was highest at 2 days after ischemia-reperfusion and was detected in microglia at this time. Thereafter, both PPARgamma immunoreactivity and protein level were decreased with time in the ischemic DG. These results indicate that PPARgamma may be related to the ischemia-induced microglial activation and neuronal damage/death in the DG after transient global cerebral ischemia.
Keyword
MeSH Terms
Figure
Reference
-
1. Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982; 239:57–69.2. Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984; 62:201–208.3. Moon JB, Lee CH, Park CW, Cho JH, Hwang IK, Yoo KY, Choi JH, Shin HC, Won MH. Neuronal degeneration and microglial activation in the ischemic dentate gyrus of the gerbil. J Vet Med Sci. 2009; 71:1381–1386.4. Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005; 26:244–251.5. Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000; 49:497–505.6. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998; 391:79–82.7. Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000; 12:2215–2223.8. Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996; 137:354–366.9. Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004; 123:131–145.10. Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK. Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem. 1993; 268:26817–26820.11. Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J Neurochem. 2002; 82:615–624.12. Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000; 20:558–567.13. Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Peroxisome proliferator-activated receptor gamma is expressed in hippocampal neurons and its activation prevents beta-amyloid neurodegeneration: role of Wnt signaling. Exp Cell Res. 2005; 304:91–104.14. Lee CH, Park OK, Yoo KY, Byun K, Lee B, Choi JH, Hwang IK, Kim YM, Won MH. The role of peroxisome proliferator-activated receptor gamma, and effects of its agonist, rosiglitazone, on transient cerebral ischemic damage. J Neurol Sci. 2011; 300:120–129.15. Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006; 97:435–448.16. Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005; 130:685–696.17. Xu F, Li J, Ni W, Shen YW, Zhang XP. Peroxisome proliferator-activated receptor-gamma agonist 15d-prostaglandin J2 mediates neuronal autophagy after cerebral ischemia-reperfusion injury. PLoS One. 2013; 8:e55080.18. Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006; 20:1162–1175.19. Wang H, Jiang R, He Q, Zhang Y, Zhang Y, Li Y, Zhuang R, Luo Y, Li Y, Wan J, Tang Y, Yu H, Jiang Q, Yang J. Expression pattern of peroxisome proliferator-activated receptors in rat hippocampus following cerebral ischemia and reperfusion injury. PPAR Res. 2012; 2012:596394.20. Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000; 106:723–731.21. Lees GJ. The possible contribution of microglia and macrophages to delayed neuronal death after ischemia. J Neurol Sci. 1993; 114:119–122.22. Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007; 61:352–362.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Brain-derived Neurotrophic Factor mRNA in Transient Cerebral Ischemic Model of Gerbil Mouse
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy
- Remifentanil alleviates transient cerebral ischemia-induced memory impairment through suppression of apoptotic neuronal cell death in gerbils
- Colorectal Cancer Expression of Peroxisome Proliferator-Activated Receptor gamma Is Associated with Good Prognosis
- Expressions of the pERK1/2 and the cFos Proteins at an Early Stage of Transient Global Ischemia-reperfusion Injury in the Hippocampus of Rats