Yonsei Med J.
2013 Sep;54(5):1178-1185. 10.3349/ymj.2013.54.5.1178.
Feasibility of Sorafenib Combined with Local Radiotherapy in Advanced Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. jsseong@yuhs.ac
- 2Yonsei Liver Cancer Special Clinic, Yonsei University Health System, Seoul, Korea.
- KMID: 1793165
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1178
Abstract
- PURPOSE
Sorafenib is an effective systemic agent for advanced hepatocellular carcinoma. To increase its efficacy, we evaluated the feasibility and benefit of sorafenib combined with radiotherapy.
MATERIALS AND METHODS
From July 2007 to July 2011, 31 patients were treated with a daily dose of 800 mg of sorafenib and radiotherapy. Among them, 13 patients who received radiotherapy on the bone metastasis were excluded. Thirteen patients received 30-54 Gy of radiotherapy on the primary tumor (primary group) and 5 patients received 30-58.4 Gy on the measurable metastatic lesions (measurable metastasis group). Tumor responses at 1 month after the completion of radiotherapy and overall survival were evaluated.
RESULTS
The in-field response rate was 100% in the primary group and 60% in the measurable metastasis group. A decrease of more than 80% in the tumor marker alpha-fetoprotein was observed in 7 patients in the primary group (54%). Toxicities of grades 3-4 were hand-foot syndrome in 3 (17%) patients, duodenal bleeding in 1 (6%) patient, thrombocytopenia in 3 (17%) patients and elevation of aspartate transaminase in 1 (6%) patient. The median overall survival was 7.8 months (95% confidence interval, 3.0-12.6).
CONCLUSION
The combined treatment of sorafenib and radiotherapy was feasible and induced substantial tumor responses in the target lesions. The results of this study emphasize the importance of individualized approach in the management of advanced hepatocellular carcinoma and encourage the initiation of a controlled clinical trial.
MeSH Terms
-
Antineoplastic Agents/administration & dosage/adverse effects/*therapeutic use
Carcinoma, Hepatocellular/drug therapy/pathology/*radiotherapy
Chemotherapy, Adjuvant
Feasibility Studies
Female
Humans
Liver Neoplasms/drug therapy/pathology/*radiotherapy
Male
Niacinamide/administration & dosage/adverse effects/*analogs & derivatives/therapeutic use
Phenylurea Compounds/administration & dosage/adverse effects/*therapeutic use
Radiation Dosage
Radiotherapy/adverse effects
Antineoplastic Agents
Niacinamide
Phenylurea Compounds
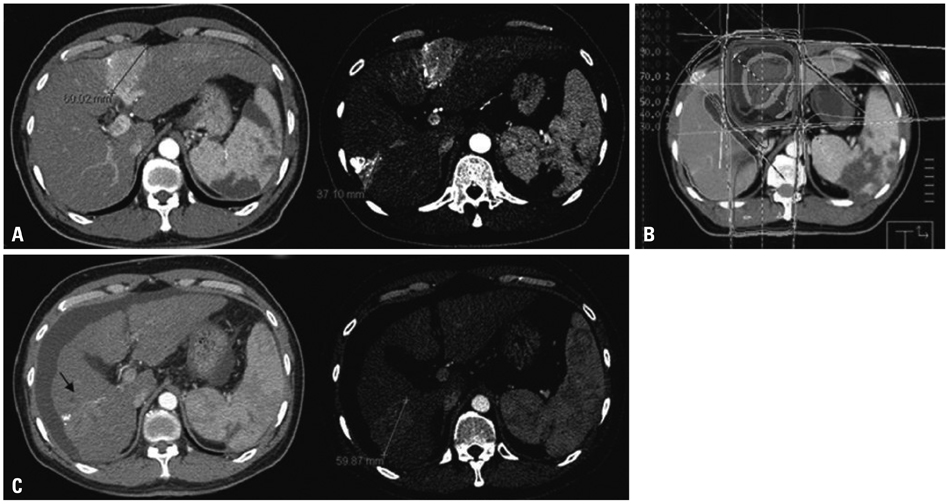
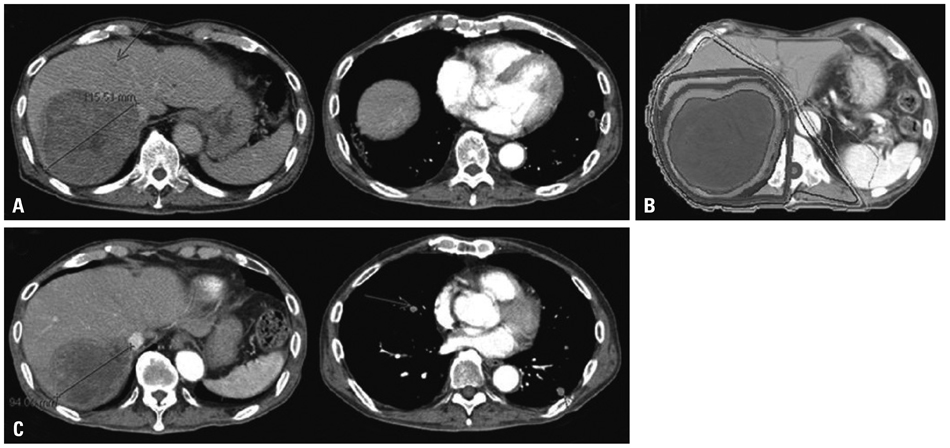
Figure
Cited by 2 articles
-
Risk Factors for Patients with Stage IVB Hepatocellular Carcinoma and Extension into the Heart: Prognostic and Therapeutic Implications
Chung Hwan Jun, Da Woon Sim, Sang Ho Kim, Hyoung Ju Hong, Min Woo Chung, Sung Bum Cho, Chang Hwan Park, Young Eun Joo, Hyun Soo Kim, Sung Kyu Choi, Jong Sun Rew
Yonsei Med J. 2014;55(2):379-386. doi: 10.3349/ymj.2014.55.2.379.Sorafenib combined with radiation therapy for advanced hepatocellular carcinoma with portal and hepatic vein invasion extending to the inferior vena cava: a complete response case according to modified RECIST criteria
Yuri Cho, Bo Hyun Kim, Tae Hyun Kim, Young Hwan Koh, Joong-Won Park
J Liver Cancer. 2022;22(1):63-68. doi: 10.17998/jlc.2022.01.18.
Reference
-
1. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132:2557–2576.
Article2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
Article3. Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999; 19:271–285.
Article4. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.
Article5. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004; 127:5 Suppl 1. S35–S50.
Article6. Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008; 23:467–473.
Article7. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109.
Article8. Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007; 59:561–574.
Article9. Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998; 27:951–958.
Article10. Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007; 27:55–76.
Article11. Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006; 130:1117–1128.
Article12. Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004; 41:864–880.
Article13. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
Article14. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article15. Zhang T, Ding X, Wei D, Cheng P, Su X, Liu H, et al. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010; 21:326–332.
Article16. National Comprehensive Cancer Network. Hepatobiliary cancers, version 2. accessed on 2012 July 10. Available at http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.17. Han KH, Seong J, Kim JK, Ahn SH, Lee do Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008; 113:995–1003.
Article18. Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005; 25:1189–1196.
Article19. Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009]. Korean J Hepatol. 2009; 15:391–423.20. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003; 38:207–215.
Article21. Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012; 118:147–156.
Article22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.
Article23. Robertson JM, McGinn CJ, Walker S, Marx MV, Kessler ML, Ensminger WD, et al. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997; 39:1087–1092.
Article24. Stillwagon GB, Order SE, Guse C, Klein JL, Leichner PK, Leibel SA, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989; 17:1223–1229.
Article25. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996; 14:722–728.
Article26. Yoon SM, Kim JH, Choi EK, Ahn SD, Lee SW, Yi BY, et al. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004; 36:79–84.
Article27. Zhou ZH, Liu LM, Chen WW, Men ZQ, Lin JH, Chen Z, et al. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 2007; 80:194–201.
Article28. Cheng JC, Chuang VP, Cheng SH, Huang AT, Lin YM, Cheng TI, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000; 47:435–442.
Article29. Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002; 54:150–155.
Article30. Wang CJ, Leung SW, Chen HC, Sun LM, Fang FM, Huang EY, et al. The correlation of acute toxicity and late rectal injury in radiotherapy for cervical carcinoma: evidence suggestive of consequential late effect (CQLE). Int J Radiat Oncol Biol Phys. 1998; 40:85–91.
Article31. Sanguineti G, Endres EJ, Parker BC, Bicquart C, Little M, Chen G, et al. Acute toxicity of whole-pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncol. 2008; 47:301–310.
Article32. Peeters ST, Heemsbergen WD, van Putten WL, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005; 61:1019–1034.
Article33. Vavassori V, Fiorino C, Rancati T, Magli A, Fellin G, Baccolini M, et al. Predictors for rectal and intestinal acute toxicities during prostate cancer high-dose 3D-CRT: results of a prospective multi-center study. Int J Radiat Oncol Biol Phys. 2007; 67:1401–1410.
Article34. Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010; 15:85–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatments Other than Sorafenib for Patients with Advanced Hepatocellular Carcinoma
- Sorafenib combined with radiation therapy for advanced hepatocellular carcinoma with portal and hepatic vein invasion extending to the inferior vena cava: a complete response case according to modified RECIST criteria
- Can metronomic chemotherapy be an alternative to sorafenib in advanced hepatocellular carcinoma?
- Complete Response of Single Nodular Large Hepatocellular Carcinoma with Pulmonary Metastasis by Sequential Transarterial Chemoembolization and Sorafenib: A Case Report
- The Efficacy of Sorafenib for Patients With Advanced Hepatocellular Carcinoma