J Korean Med Sci.
2008 Aug;23(4):586-591. 10.3346/jkms.2008.23.4.586.
Phase II Study of Paclitaxel, Cisplatin, and 5-Fluorouracil Combination Chemotherapy in Patients with Advanced Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Division of Hemato/Oncology, Chonnam National University Medical School, Gwangju, Korea. ijchung@chonnam.ac.kr
- KMID: 1785814
- DOI: http://doi.org/10.3346/jkms.2008.23.4.586
Abstract
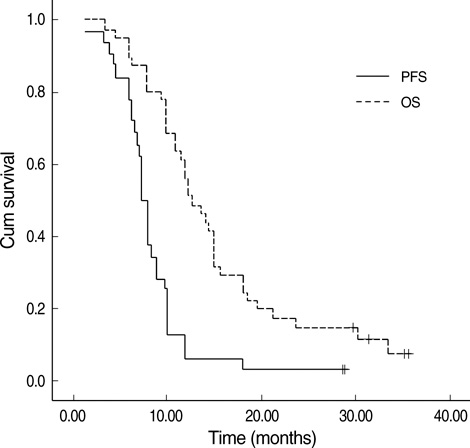
- This phase II study evaluated the efficacy and safety of combination chemotherapy with paclitaxel, cisplatin, and 5-fluorouracil (5-FU) in advanced gastric cancer. Patients with histologically confirmed gastric adenocarcinoma were eligible for the study. Paclitaxel (175 mg/m(2)) and cisplatin (75 mg/m(2)) were given as a 1-hr intravenous infusion on day 1, followed by 5-FU (750 mg/m(2)) as a 24-hr continuous infusion for 5 days. This cycle was repeated every 3 weeks. Forty-five eligible patients (median age, 56 yr) were treated in this way. Of the 41 patients in whom efficacy was evaluable, an objective response rate (ORR) was seen in 51.2% (95% CI, 0.35-0.67), a complete response in two, and a partial response in 19 patients. The median progression free survival was 6.9 months (95% CI, 5.86-7.94 months), and the median overall survival was 12.7 months (95% CI, 9.9-15.5). The main hematological toxicity was neutropenia and greater than grade 3 neutropenia was observed in twelve patients (54%). Febrile neutropenia developed in three patients (6.8%). The major non-hematological toxicities were asthenia and peripheral neuropathy, but most of patients showed grade 1 or 2. In conclusion, combination chemotherapy with paclitaxel, cisplatin, and 5-FU is a promising regimen, and was well tolerated in patients with advanced gastric cancer.
Keyword
MeSH Terms
-
Adenocarcinoma/*drug therapy/mortality
Adult
Aged
Antineoplastic Combined Chemotherapy Protocols/*therapeutic use
Cisplatin/administration & dosage
Disease-Free Survival
Female
Fluorouracil/administration & dosage
Humans
Male
Middle Aged
Paclitaxel/administration & dosage
Stomach Neoplasms/*drug therapy/mortality
Figure
Reference
-
1. Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003. 14:Suppl 2. ii31–ii36.
Article2. Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993. 72:37–41.
Article3. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with nonresectable gastric cancer. Br J Cancer. 1995. 71:587–591.
Article4. Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H, Heuman R. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997. 8:163–168.
Article5. Comis RL, Carter SK. A review of chemotherapy in gastric cancer. Cancer. 1974. 34:1576–1586.
Article6. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.
Article7. Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK, Shin DB, Kim HT, Kim HJ. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993. 71:3813–3818.
Article8. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979. 277:665–667.
Article9. Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxnes-induced apoptosis. Curr Med Chem Anticancer Agents. 2003. 3:291–306.10. Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. A Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999. 22:580–586.11. Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999. 85:295–301.
Article12. Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, Morant R, Borner MM, Herrmann R, Honegger H, Cavalli F, Alberto P, Castiglione M, Goldhirsch A. Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO). Ann Oncol. 2000. 11:301–306.13. Constenla M, Garcia-Arroyo R, Lorenzo I, Carrete N, Campos B, Palacios P. Docetaxel, 5-fluorouracil, and leucovorin as treatment for advanced gastric cancer: results of a phase II study. Gastric cancer. 2002. 5:142–147.
Article14. Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, Majlis A, Assadourian S, Van Cutsem E. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastoesophageal adenocarcinoma. J Clin Oncol. 2005. 23:5660–5667.15. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA; V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol. 2006. 24:4991–4997.
Article16. Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, Parkin D, Paul J, Hay A, Kaye SB; Scottish Gynaecological Cancer Trials Group. Phase III randomized trial of docetaxel-carboplain versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004. 96:1682–1691.17. Hsu Y, Sood AK, Sorosky JI. Docetaxel versus paclitaxel for adjuvant treatment of ovarian cancer: case-control analysis of toxicity. Am J Clin Oncol. 2004. 27:14–18.18. Park SH, Lee WK, Chung M, Lee Y, Han SH, Bang SM, Cho EK, Shin DB, Lee JH. Paclitaxel versus docetaxel for advanced gastric cancer: a randomized phase II trial in combination with infusional 5-fluorouracil. Anticancer Drugs. 2006. 17:225–229.
Article19. Cascinu S, Graziano F, Cardarelli N, Marcellini M, Giordani P, Menichetti ET, Catalano G. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs. 1998. 9:307–310.
Article20. Sulkes A, Smyth J, Sessa C, Dirix LY, Vermorken JB, Kaye S, Wanders J, Franklin H, LeBail N, Verweij J. Docetaxel (Taxotere) in advanced gastric cancer: results of a phase II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer. 1994. 70:380–383.21. Einzig AI, Neuberg D, Remick SC, Karp DD, O'Dwyer PJ, Stewart JA, Benson AB 3rd. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: the Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996. 13:87–93.
Article22. Rowinsky EK, Gilbert MR, McGuire WP, Noe DA, Grochow LB, Forastiere AA, Ettinger DS, Lubejko BG, Clark B, Sartorius SE. Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991. 9:1692–1703.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase II Trial of Paclitaxel, 5-fluorouracil (5-FU) and Cisplatin in Patients with Metastatic or Recurrent Gastric Cancer
- Combination chemotherapy with 5-fluorouracil and cisplatin for advanced gastric cancer
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Chemotherapy of Advanced Gastric Cancer
- A Phase II Study of Paclitaxel and Cisplatin as Salvage Therapy for Patients with Advanced or Metastatic Gastric Cancer