J Periodontal Implant Sci.
2010 Apr;40(2):49-55. 10.5051/jpis.2010.40.2.49.
The influence of diabetes mellitus on periodontal tissues: a pilot study
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Department of Oral Biology, BK21 Project, Oral Science Research Center, and Research Center for Orofacial Hard Tissue Regeneration, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 1783534
- DOI: http://doi.org/10.5051/jpis.2010.40.2.49
Abstract
- PURPOSE
The purpose of this study was to preliminarily evaluate the influence of diabetes mellitus (DM) on periodontal tissue without establishment of periodontitis.
METHODS
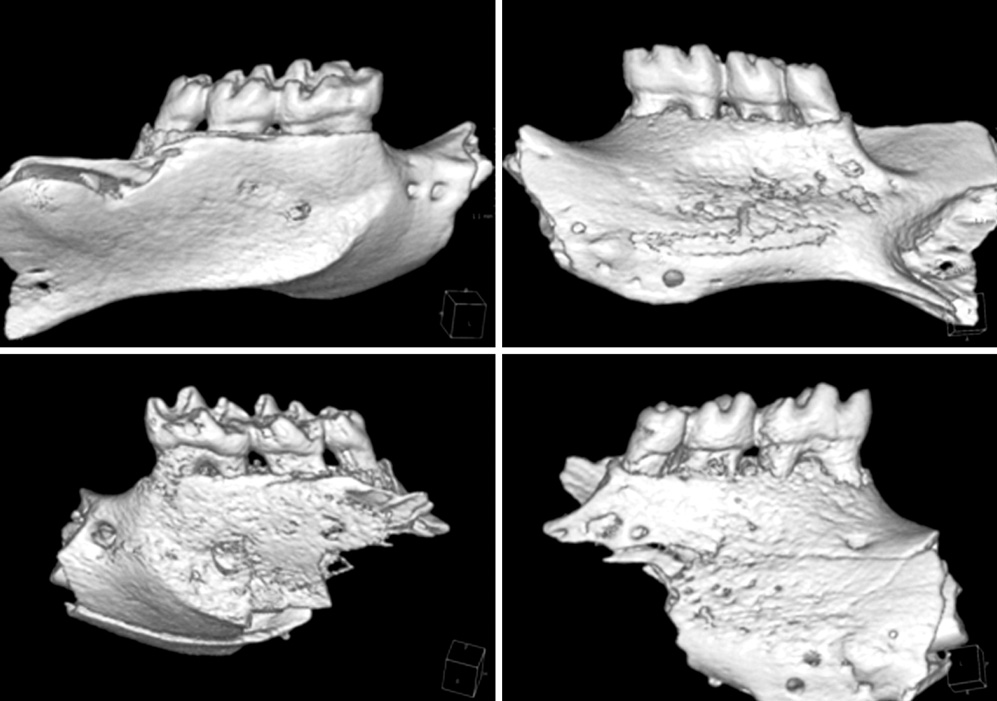
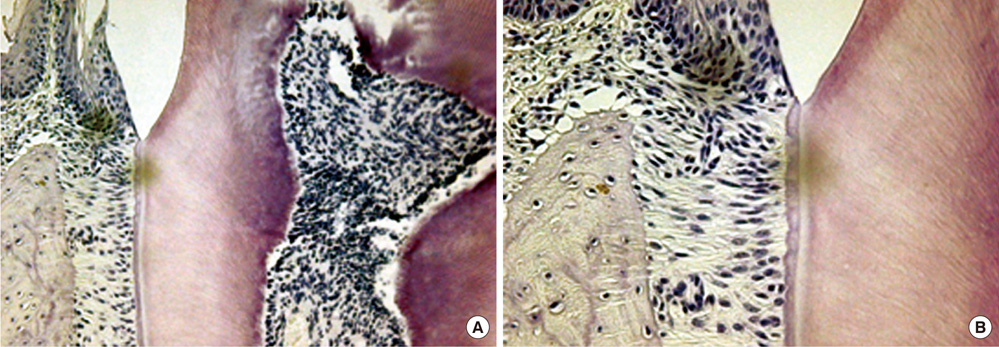
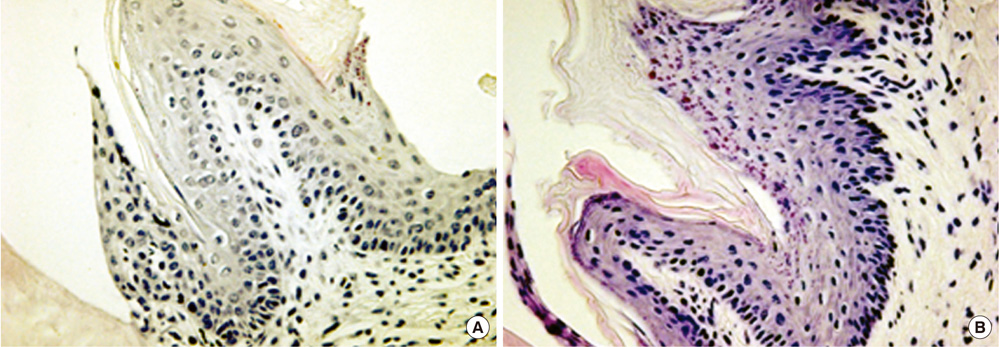
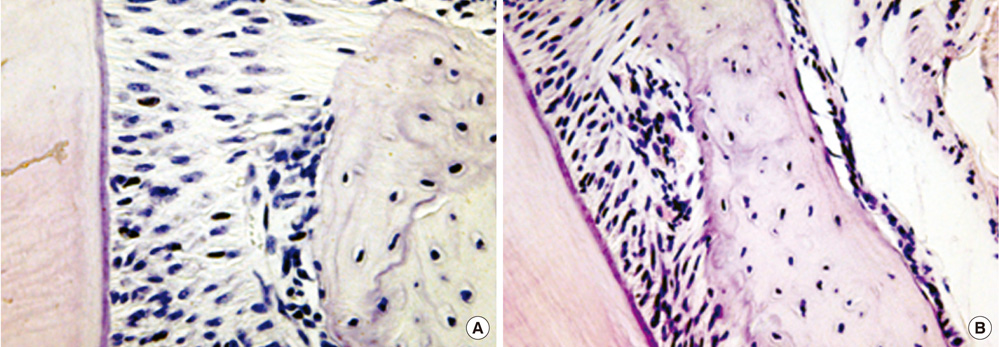
Seven-week-old db/db mice were used for the diabetic experimental group and systematically healthy mice of the same age were used as controls. After 1 week of acclimatization, the animals were sacrificed for hard and soft tissue evaluation. The pattern of bone destruction was evaluated by stereomicroscope evaluation with alizarin red staining and radiographic evaluation by microscopic computerized tomography images. Histological evaluation was performed with hematoxylin and eosin stain for evaluation of soft tissue changes.
RESULTS
In both stereomicroscope evaluation and radiograph image analysis, aggressive form of bone destruction was observed in diabetic animals when compared to the systematically healthy controls. In histological evaluation, apical migration of junctional epithelium with slight inflammatory cell infiltration was observed with disarrangement of connective tissue fibers.
CONCLUSIONS
Within the limits of this study, diabetic animals presented distortion in periodontal attachment and an aggressive bone loss pattern when compared to the healthy controls, suggesting that DM has an independent effect on periodontal tissue destruction irrespective of the presence or absence of periodontal disease.
Keyword
MeSH Terms
Figure
Reference
-
1. Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007. 44:127–153.
Article2. Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990. 121:532–536.
Article3. Mealey BL. Periodontal implications: medically compromised patients. Ann Periodontol. 1996. 1:256–321.
Article4. Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996. 1:1–36.
Article5. Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991. 62:123–131.
Article6. Bartolucci EG, Parkes RB. Accelerated periodontal breakdown in uncontrolled diabetes: pathogenesis and treatment. Oral Surg Oral Med Oral Pathol. 1981. 52:387–390.7. Nassar H, Kantarci A, van Dyke TE. Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontol 2000. 2007. 43:233–244.
Article8. Nishimura F, Iwamoto Y, Soga Y. The periodontal host response with diabetes. Periodontol 2000. 2007. 43:245–253.
Article9. Graves DT, Liu R, Oates TW. Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathosis. Periodontol 2000. 2007. 45:128–137.
Article10. Lalla E. Periodontal infections and diabetes mellitus: when will the puzzle be complete? J Clin Periodontol. 2007. 34:913–916.
Article11. Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000. 49:22–31.
Article12. Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992. 63:322–331.
Article13. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol 2000. 2006. 40:11–28.
Article14. Silva JA, Lorencini M, Reis JR, Carvalho HF, Cagnon VH, Stach-Machado DR. The influence of type I diabetes mellitus in periodontal disease induced changes of the gingival epithelium and connective tissue. Tissue Cell. 2008. 40:283–292.
Article15. Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996. 67:1085–1093.
Article16. Bouillon R. Diabetic bone disease. Calcif Tissue Int. 1991. 49:155–160.
Article17. Kawaguchi H, Kurokawa T, Hanada K, Hiyama Y, Tamura M, Ogata E, et al. Stimulation of fracture repair by recombinant human basic fibroblast growth factor in normal and streptozotocin-diabetic rats. Endocrinology. 1994. 135:774–781.
Article18. Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003. 144:346–352.
Article19. Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC. A role for advanced glycation end products in diminished bone healing in type 1 diabetes. Diabetes. 2003. 52:1502–1510.
Article20. Ohnishi T, Bandow K, Kakimoto K, Machigashira M, Matsuyama T, Matsuguchi T. Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type 2 diabetes. J Periodontal Res. 2009. 44:43–51.
Article21. Sznajder N, Carraro JJ, Rugna S, Sereday M. Periodontal findings in diabetic and nondiabetic patients. J Periodontol. 1978. 49:445–448.
Article22. Sakallioglu EE, Ayas B, Lutfioglu M, Keles GC, Acikgoz G, Firatli E. Gingival levels of monocyte chemoattractant protein-1 (MCP-1) in diabetes mellitus and periodontitis: an experimental study in rats. Clin Oral Investig. 2008. 12:83–89.
Article23. Emingil G, Atilla G, Huseyinov A. Gingival crevicular fluid monocyte chemoattractant protein-1 and RANTES levels in patients with generalized aggressive periodontitis. J Clin Periodontol. 2004. 31:829–834.
Article24. Garlet GP, Martins W Jr., Ferreira BR, Milanezi CM, Silva JS. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodontal Res. 2003. 38:210–217.
Article25. Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000. 121:437–443.
Article26. Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, et al. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med. 2004. 21:1292–1297.
Article