Korean J Urol.
2009 Aug;50(8):805-811. 10.4111/kju.2009.50.8.805.
Effect of Mitomycin-C on Experimentally Induced Urethral Stricture in Rats
- Affiliations
-
- 1Department of Urology, College of Medicine, Chosun University, Gwangju, Korea. mu-hn@hanmail.net
- KMID: 1780486
- DOI: http://doi.org/10.4111/kju.2009.50.8.805
Abstract
- PURPOSE
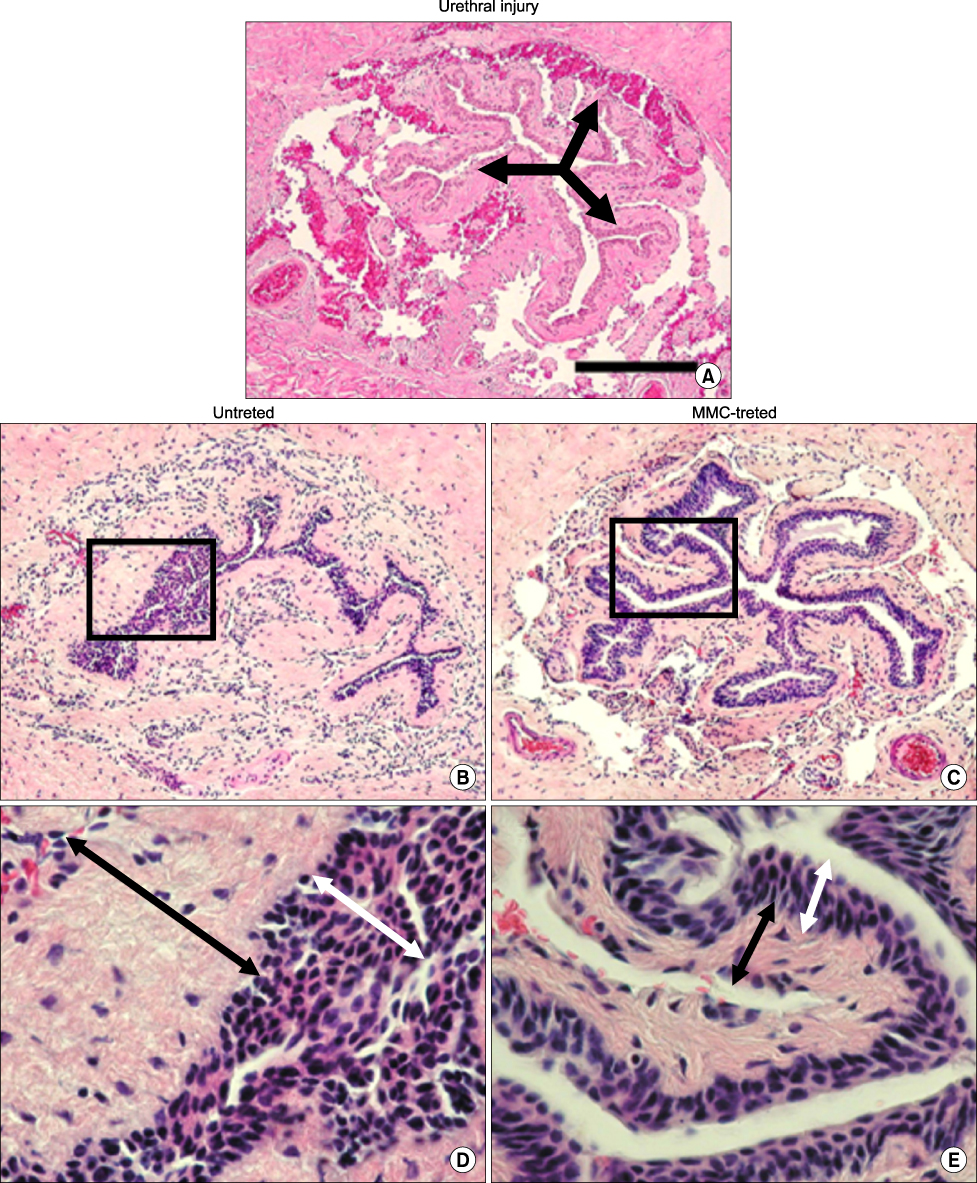
Urethral stricture is a fibrotic process and, although it is one of the oldest known urologic diseases, it remains a common problem with a high recurrence rate. Mitomycin-C has been reported to have anti-replication properties in animal and clinical studies. The aim of this study was to observe the intraurethral impact of the use of mitomycin-C on scar formation and fibrosis in an experimental rat model of urethral stricture. MATERIALS AND METHODS: Twelve male Sprague-Dawley rats were used. With the animals under deep anesthesia, an internal urethrotomy was made with a cold knife. After the urethral injury, the rats were grouped randomly as follows: group 1 (control, n=4), group 2 (3 mg/l of mitomycin-C, n=4), and group 3 (5 mg/l of mitomycin-C, n=4). The rats were sacrificed 14 days later to evaluate epithelial proliferation and fibrosis. The penile urethra was removed and histopathologically examined by H&E staining, Masson trichrome staining, and immunohistochemistry with anti-collagen type I antibody. RESULTS: The stained specimens were examined under a light microscope. The extent of fibrosis and re-epithelization after urethral injury was greater in the areas with trauma than in those without. These findings were significantly reduced in the groups treated with mitomycin-C as compared with group 1, but there was no statistical difference between group 2 and group 3. Mitomycin-C treatment also prevented increases in collagen type I, whereas group 1 showed increases in collagen type I and collagen contents at the stricture site. CONCLUSIONS: These results suggest that mitomycin-C might inhibit the renewal of the epithelium and the synthesis of collagen secreted by fibroblasts in the affected urethra and then prevent scar formation. This raises the possibility of the use of mitomycin-C to prevent urethral stricture caused by trauma.
Keyword
MeSH Terms
Figure
Reference
-
1. Jordan GH, Schilossberg SM. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Surgery of the penis and urethra. Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;1023–1097.2. Fenton AS, Morey AF, Aviles R, Garcia CR. Anterior urethral strictures: etiology and characteristics. Urology. 2005. 65:1055–1058.3. Conort P, Chartier-Kastler E, Richard F, Chatelain C. Secondary management of urethral injuries. Chirurgie. 1996. 121:372–376.4. Scott TM, Foote J. Early events in stricture formation in the guinea pig urethra. Urol Int. 1980. 35:334–339.5. Kim ST, Lee BY, Ko SH, Chung YS, Cho CH, Park JS, et al. The effect of mitomycin C in middle meatal antrostomy site. Korean J Otolaryngol-Head Neck Surg. 2002. 45:585–588.6. Choi HS, Lim JY, Kim HS, Hong HJ, Yoo JB, Kim JH, et al. Preliminary results of mitomycin-C application in the treatment of laryngeal stenosis and granuloma. Korean J Otolaryngol-Head Neck Surg. 2003. 46:508–512.7. Kao SC, Liao CL, Tseng JH, Cheng MS, Hou PK. Dacryocystorhinostomy with intraoperative mitomycin C. Ophthalmology. 1997. 104:86–91.8. Bradner WT. Mitomycin C: a clinical update. Cancer Treat Rev. 2001. 27:35–50.9. Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992. 19:573–580.10. Ayyildiz A, Nuhoglu B, Gulerkaya B, Caydere M, Ustun H, Germiyanoglu C, et al. Effect of intraurethral Mitomycin-C on healing and fibrosis in rats with experimentally induced urethral stricture. Int J Urol. 2004. 11:1122–1126.11. McAninch JW, Laing FC, Jeffrey RB Jr. Sonourethrography in the evaluation of urethral strictures: a preliminary report. J Urol. 1988. 139:294–297.12. Huh G, Jung GW, Yoon JH. Clinical assessment of visual internal urethrotomy as primary treatment of urethral stricture. Korean J Urol. 1996. 37:798–803.13. Chung YC, Kim BH, Chang HS, Park CH, Kim CI. Clinical evaluation of repeated internal urethrotomy in incomplete anterior urethral stricture. Korean J Urol. 2004. 45:919–923.14. Webster GD, Koefoot RB, Sihelnik SA. Urethroplasty management in 100 cases of urethral stricture: a rationale for procedure selection. J Urol. 1985. 134:892–898.15. Gonzalez R, Chiou RK, Hekmat K, Fraley EE. Endoscopic reestablishment of urethral continuity after traumatic disruption of the membranous urethra. J Urol. 1983. 130:785–787.16. Marshall FF. Endoscopic reconstruction of traumatic urethral transections. Urol Clin North Am. 1989. 16:313–318.17. Kim YJ, Lee CH, Lee SJ. Therapeutic effect of internal urethrotomy in post-traumatic complete urethral stricture. Korean J Urol. 2002. 43:1061–1064.18. Meria P, Anidjar M, Brouland JP, Teillac P, Berthon P, Cussenot O. Gene transfer to urethral strictures in rabbits: a preliminary report. BJU Int. 2000. 85:1120–1125.19. Hebert PW. The treatment of urethral stricture: transurethral injection of triamcinolone. J Urol. 1972. 108:745–747.20. Estrem SA, Vanleeuwen RN. Use of mitomycin C for maintaining myringotomy patency. Otolaryngol Head Neck Surg. 2000. 122:8–10.21. Chung JH, Cosenza MJ, Rahbar R, Metson RB. Mitomycin C for the prevention of adhesion formation after endoscopic sinus surgery: a randomized, controlled study. Otolaryngol Head Neck Surg. 2002. 126:468–474.22. Roh JL, Koo BS, Yoon YH, Rha KS, Park CI. Effect of topical mitomycin C on the healing of surgical and laser wounds: a hint on clinical application. Otolaryngol Head Neck Surg. 2005. 133:851–856.23. Cavalcanti AG, Costa WS, Baskin LS, McAninch JA, Sampaio FJ. A morphometric analysis of bulbar urethral strictures. BJU Int. 2007. 100:397–402.24. Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol. 2007. 51:1089–1092.25. Nagler A, Gofrit O, Ohana M, Pode D, Genina O, Pines M. The effect of halofuginone, an inhibitor of collagen type I synthesis, on urethral stricture formation: in vivo and in vitro study in a rat model. J Urol. 2000. 164:1776–1780.26. Lopes JF, Schned A, Ellsworth PI, Cendrom M. Histological analysis of urethral healing after tubularized incised plate urethroplasty. J Urol. 2001. 166:1014–1017.27. Soloway MS, Perito PE. Superficial bladder cancer: diagnosis, surveillance and treatment. J Cell Biochem Suppl. 1992. 16I:120–127.28. Brady JD, Assimos DG, Jordan GH. Urethral slough: a rare and previously unreported complication of intravesical mitomycin. J Urol. 2000. 164:1305.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Modified Cecil's Operation for Urethral Stricture
- Adjuvant Value of Spongiosography in the Patients with Urethral Stricture
- The Experience of Indwelling Nylon(#1-0) for the Guidance of Managing Urethral Stricture under the Internal Urethrotomy
- Efficacy of mitomycin C in reducing recurrence of anterior urethral stricture after internal optical urethrotomy
- The Study on the Ultrasonographic Urethrogram of the Urethral Stricture