J Korean Med Sci.
2006 Jun;21(3):478-484. 10.3346/jkms.2006.21.3.478.
Disease-specific Proteins from Rheumatoid Arthritis Patients
- Affiliations
-
- 1Department of Biochemistry and RINS, College of Medicine and Institute of Health Sciences, Gyeongsang National University, Jinju, Korea. drkim@gsnu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 1778432
- DOI: http://doi.org/10.3346/jkms.2006.21.3.478
Abstract
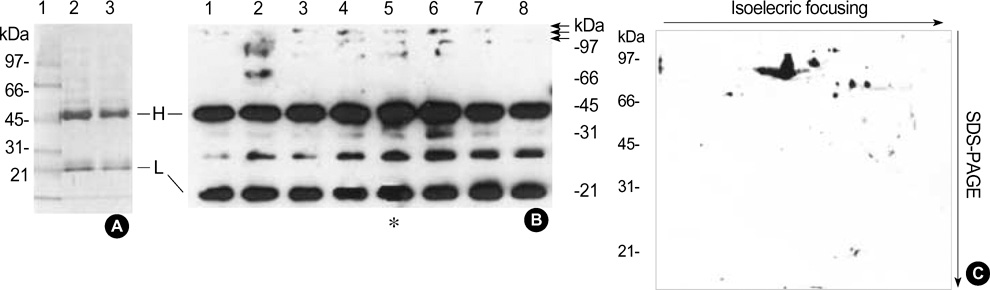
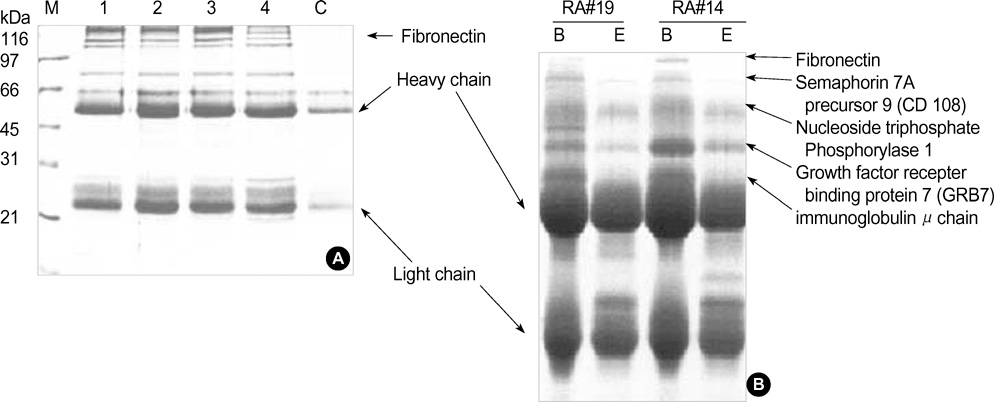
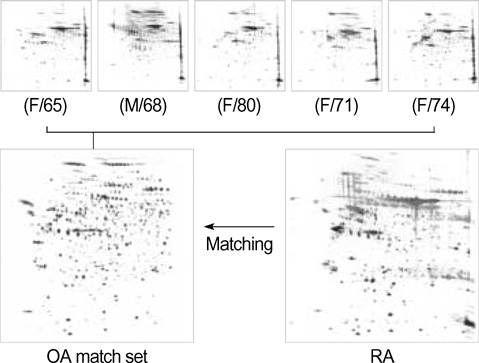
- Rheumatoid arthritis (RA) is a chronic inflammatiory disease that mainly destroys cartilages or bones at the joints. This inflammatory disorder is initiated by self-attack using own immune system, but the detail of pathological mechanism is unclear. Features of autoantigens leading to autoimmune disease are also under veil although several candidates including type II collagen have been suggested to play a role in pathogenesis. In this report, we tried to identify proteins responding to antibodies purified from RA patients and screen proteins up-regulated or down-regulated in RA using proteomic approach. Fibronectin, semaphorin 7A precursor, growth factor binding protein 7 (GRB7), and immunoglobulin mu chain were specifically associated with antibodies isolated from RA synovial fluids. In addition, some metabolic proteins such as adipocyte fatty acid binding protein, galectin-1 and apolipoprotein A1 precursor were overexpressed in RA synovium. Also, expression of peroxiredoxin 2 was up-regulated in RA. On the contrary, expression of vimentin was severely suppressed in RA synoviocytes. Such findings might give some insights into understanding of pathological mechanism in RA.
Keyword
MeSH Terms
Figure
Reference
-
1. Ziff M. Rheumatoid arthritis-Its present and future. J Rheumatol. 1990. 17:127–133.2. Janossy G, Panayi G, Duke O, Bofill M, Poulter LW, Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981. 2:839–842.
Article3. Cush JJ, Lipsky PE. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988. 31:1230–1238.
Article4. Yamanishi Y, Firestein GS. Pathogenesis of rheumatoid arthritis: the role of synoviocytes. Rheum Dis Clin North Am. 2001. 27:335–371.
Article5. Feldmann M, Brennan FM, Mainin RN. Rheumatoid arthritis. Cell. 1996. 85:307–310.
Article6. Schaller M, Burton DR, Ditzel HJ. Autoantibodies to GPI rheumatoid arthritis: linakage between and animal model and human disease. Nat Immunol. 2001. 2:746–753.7. Holmdahi R, Andersson M, Goldschmidt TJ, Gustafasson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990. 118:193–232.
Article8. Kim DR. Proteomic changes during the B cell development. J Chromatogr B Analyt Technol Biomed Life Sci. 2005. 815:295–303.
Article9. Jin SH, Cho EH, Ko JE, Jung EH, Ahn B, Hahm JR, Kim JW, Kim CW, Kim DR. Comparative analysis of nuclear proteins of B cells in different developmental stages. Proteomics. 2003. 3:2428–2436.
Article10. Hwa JS, Park HJ, Jung JH, Kam SC, Park HC, Kim CW, Kang KR, Hyun JS, Chung KH. Identification of proteins differentially expressed in the conventional renal cell carcinoma by proteomic analysis. J Korean Med Sci. 2005. 20:450–455.
Article11. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001. 344:907–916.
Article12. Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J Immunol. 2002. 169:1459–1466.13. Vos K, Miltenburg AM, van Meijgaarden KE, van den Heuvel M, Elferink DG, van Galen PJ, van Hogezand RA, van Vliet-Daskalopoulou E, Ottenhoff TH, Breedveld FC, Boots AM, de Vries RR. Cellular immune response to human cartilage glycoprotein-39 (HCgp-39)-derived peptides in rheumatoid arthritis and other inflammatory conditions. Rheumatolology. 2000. 39:1326–1331.14. Patil NS, Hall FC, Drover S, Spurrell DR, Bos E, Cope AP, Sonderstrup G, Mellins ED. Autoantigenic HCgp39 epitopes are presented by the HLA-DM-dependent presentation pathway in human B cells. J Immunol. 2001. 166:33–41.
Article15. Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, Mathis D, Benoist C. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002. 3:360–365.
Article16. Manicourt DH, Poilvache P, Van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ. Synovial fluid levels of tumor necrosis factor alpha and oncostatin M correlate with levels of markers of the degradation of crosslinked collagen and cartilage aggrecan in rheumatoid arthritis but not in osteoarthritis. Arthritis Rheum. 2000. 43:281–288.17. Union A, Meheus L, Humbel RL, Conrad K, Steiner G, Moereels H, Pottel H, Serre G, De Keyser F. Identification of citrullinated rheumatoid arthritis-specific epitopes in natural filaggrin relevant for antifilaggrin autoantibody detection by line immunoassay. Arthritis Rheum. 2002. 46:1185–1195.
Article18. Corrigall VM, Bodman-Smith MD, Fife MS, Canas B, Myers LK, Wooley P, Soh C, Staines NA, Pappin DJ, Berlo SE, van Eden W, van Der Zee R, Lanchbury JS, Panayi GS. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001. 166:1492–1498.
Article19. Blass S, Union A, Raymackers J, Schumann F, Ungethum U, Muller-Steinbach S, De Keyser F, Engel JM, Burmester GR. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 2001. 44:761–771.20. Shiozawa K, Hino K, Shiozawa S. Alternatively spliced EDA-containing fibronectin in synovial fluid as a predictor of rheumatoid joint destruction. Rheumatology. 2001. 40:739–742.
Article21. Claudepierre P, Allanore Y, Belec L, Larget-Piet B, Zardi L, Chevalier X. Increased Ed-B fibronectin plasma levels in spondyloarthropathies: comparison with rheumatoid arthritis patients and a healthy population. Rheumatology. 1999. 38:1099–1103.
Article22. Bing DH, Almeda S, Isliker H, Lahav J, Hynes RO. Fibronectin binds to the C1q component of complement. Proc Nat Acad Sci USA. 1982. 79:4198–4201.
Article23. Rittling SR, Baserga R. Functional analysis and growth factor regulation of the human vimentin promoter. Mol Cell Biol. 1987. 7:3908–3915.
Article24. Franke WW, Schmid E, Winter S, Osborn M, Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979. 123:25–46.
Article25. Hendrix MJ, Seftor EA, Chu YW, Trevor KT, Seftor RE. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996. 15:507–525.
Article26. Skalli O, Goldman RD. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991. 19:67–79.
Article27. Ohshima S, Kuchen S, Seemayer CA, Kyburz D, Hirt A, Klinzing S, Michel BA, Gay RE, Liu FT, Gay S, Neidhart M. Galectin 3 and its binding protein in rheumatoid arthritis. Arthritis Rheum. 2003. 48:2788–2795.
Article28. Harjacek M, Diaz-Cano S, De Miguel M, Wolfe H, Maldonado CA, Rabinovich GA. Expression of galectins-1 and -3 correlates with defective mononuclear cell apoptosis in patients with juvenile idiopathic arthritis. J Rheumatol. 2001. 28:1914–1922.29. Karasawa R, Ozaki S, Nishioka K, Kato T. Autoantibodies to peroxiredoxin I and IV in patients with systemic autoimmune diseases. Microbiol Immunol. 2005. 49:57–65.
Article30. Bresnihan B, Gogarty M, FitzGerald O, Dayer JM, Burger D. Apolipoprotein A-I infiltration in rheumatoid arthritis synovial tissue: a control mechanism of cytokine production. Arthritis Res Ther. 2004. 6:563–566.31. Ananth L, Prete PE, Kashyap ML. Apolipoproteins A-I and B and cholesterol in synovial fluid of patients with rheumatoid arthritis. Metabolism. 1993. 42:803–806.
Article32. Zborovskii AB, Gontar IP, Emel'ianova OI, Bondarenko AV, Sycheva GF. Antibodies to skeletal muscle myofibrillar proteins in the diagnosis of rheumatoid arthritis. Ter Arkh. 1995. 67:54–56.33. Liao H, Wu J, Kuhn E, Chin W, Chang B, Jones MD, O'Neil S, Clauser KR, Karl J, Hasler F, Roubenoff R, Zolg W, Guild BC. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. 2004. 50:3792–3803.
Article