J Korean Med Sci.
2009 Jan;24(Suppl 1):S135-S142. 10.3346/jkms.2009.24.S1.S135.
Clinical Significance of Monitoring Circulating CD4+CD25+ Regulatory T Cells in Kidney Transplantation during the Early Posttransplant Period
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University, Seoul, Korea.
- 2Department of Laboratory Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Catholic Transplantation Research Center, The Catholic University of Korea, Seoul, Korea.
- 4Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea. yangch@catholic.ac.kr
- 5Department of Surgery, The Catholic University of Korea, Seoul, Korea.
- KMID: 1778153
- DOI: http://doi.org/10.3346/jkms.2009.24.S1.S135
Abstract
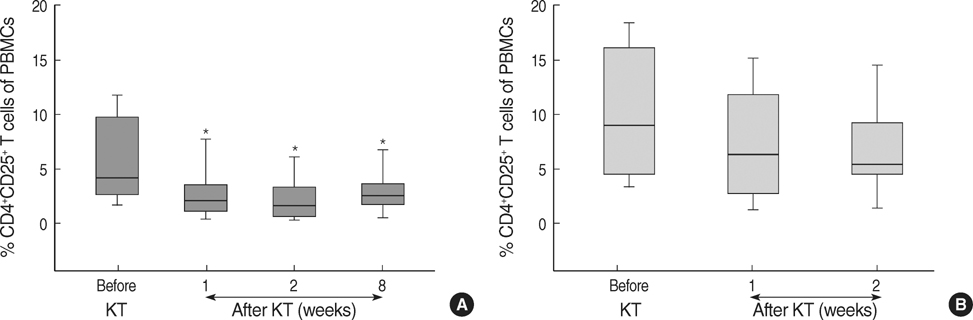
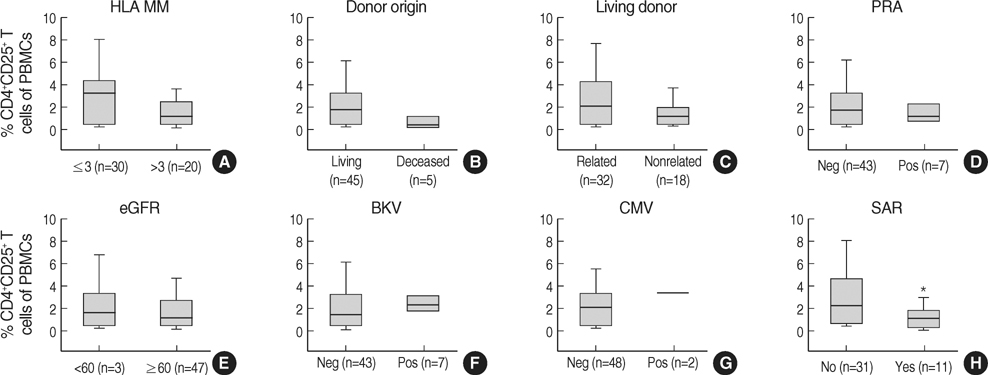
- The CD4+CD25+ T regulatory cells (Tregs) play an important role in immune tolerance in experimental transplantation but the clinical significance of circulating Tregs in the peripheral blood is undetermined. In 50 kidney transplant (KT) recipients, 29 healthy controls and 32 liver transplant (LT) recipients, the frequency of Tregs was measured with flow cytometry before and after transplantation. In the KT recipients, IL-10 secretion was measured with an enzyme-linked immunospot (ELISPOT) assay. The median frequency of circulating Tregs before KT was similar to that in healthy controls but significantly lower than that in LT patients before transplantation. The frequency of Tregs was significantly decreased in patients with subclinical acute rejection compared with those without subclinical acute rejection. Calcineurin inhibitors (CNIs) and anti-CD25 antibody decreased the frequency of Tregs but mTOR inhibitor did not. The frequency of donor-specific IL-10 secreting cells did not correlate with the number of Tregs. The frequency of circulating Tregs in KT recipients is strongly affected by CNIs and anti-CD25 antibody, and a low frequency of Tregs is associated with subclinical acute rejection during the early posttransplant period.
Keyword
MeSH Terms
-
Adult
CD4-Positive T-Lymphocytes/*immunology
Enzyme-Linked Immunosorbent Assay
Female
Flow Cytometry
Graft Rejection
Humans
Interleukin-10/metabolism
Interleukin-2 Receptor alpha Subunit/*biosynthesis
Kidney Failure, Chronic/blood/immunology/*therapy
Kidney Transplantation/*methods
Male
Middle Aged
Nephrology/*methods
T-Lymphocytes, Regulatory/*immunology
Figure
Reference
-
1. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995. 155:1151–1164.2. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001. 193:1295–1302.
Article3. Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001. 193:1285–1294.
Article4. von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005. 6:338–344.
Article5. Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003. 81:331–371.
Article6. Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002. 168:1080–1086.
Article7. Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003. 3:199–210.
Article8. Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002. 195:1641–1646.
Article9. Sakaguchi S, Sakaguchi N. Regulatory T cells in immunologic self-tolerance and autoimmune disease. Int Rev Immunol. 2005. 24:211–226.
Article10. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation. 2004. 78:242–249.
Article11. Kim SH, Oh EJ, Kim MJ, Park YJ, Han K, Yang HJ, Kim JY, Choi BS, Yang CW, Kim YS, Bang BK. Pretransplant donor-specific interferon-gamma ELISPOT assay predicts acute rejection episodes in renal transplant recipients. Transplant Proc. 2007. 39:3057–3060.12. Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, Aiello S, Cassis L, Gotti E, Gaspari F, Cattaneo D, Perico N, Remuzzi G. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol. 2007. 18:1007–1018.
Article13. Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005. 41:771–778.
Article14. Schleifer SJ, Benton T, Keller SE, Dhaibar Y. Immune measures in alcohol-dependent persons with minor health abnormalities. Alcohol. 2002. 26:35–41.
Article15. Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007. 27:194–213.
Article16. Stenard F, Nguyen C, Cox K, Kambham N, Umetsu DT, Krams SM, Esquivel CO, Martinez OM. Decreases in circulating CD4(+)CD25(hi)FOXP3(+) cells and increases in intragraft FOXP3(+) cells accompany allograft rejection in pediatric liver allograft recipients. Pediatr Transplant. 2008. [Epub ahead of print].17. Demirkiran A, Kok A, Kwekkeboom J, Kusters JG, Metselaar HJ, Tilanus HW, van der Laan LJ. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 2006. 12:277–284.
Article18. Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001. 166:3789–3796.
Article19. Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008. 14:88–92.
Article20. Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000. 164:183–190.
Article21. Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998. 188:287–296.
Article22. Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006. 108:390–399.
Article23. Baan CC, van der Mast BJ, Klepper M, Mol WM, Peeters AM, Korevaar SS, Balk AH, Weimar W. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation. 2005. 80:110–117.
Article24. Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006. 108:390–399.25. Boschiero L, Nacchia F, Fior F, Cordiano C, Tridente G, Bellisola G. Specific alloantigen self-control by regulatory T cells in organ transplantation: a review. Transplant Proc. 2007. 39:2013–2017.
Article26. Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005. 105:4743–4748.
Article27. Aramaki O, Inoue F, Takayama T, Shimazu M, Kitajima M, Ikeda Y, Okumura K, Yagita H, Shirasugi N, Niimi M. Interleukin-10 but not transforming growth factor-beta is essential for generation and suppressor function of regulatory cells induced by intratracheal delivery of alloantigen. Transplantation. 2005. 79:568–576.28. van den Boogaardt DE, van Miert PP, de Vaal YJ, de Fijter JW, Claas FH, Roelen DL. The ratio of interferon-gamma and interleukin-10 producing donor-specific cells as an in vitro monitoring tool for renal transplant patients. Transplantation. 2006. 82:844–848.29. Wood KJ, Luo S, Akl A. Regulatory T cells: potential in organ transplantation. Transplantation. 2004. 77:1 Suppl. S6–S8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- Regulatory T Cells and Allogeneic Transplantation
- Distribution of CD4+CD25+ T cells and graft-versus-host disease in human hematopoietic stem cell transplantation
- IL-4 Induces CD4+CD25+ Regulatory T Cells from CD4+CD25- T Cells in Peripheral Blood