J Korean Med Sci.
2013 May;28(5):687-692. 10.3346/jkms.2013.28.5.687.
Safety of Megestrol Acetate in Palliating Anorexia-Cachexia Syndrome in Patients with Castration-Resistant Prostate Cancer
- Affiliations
-
- 1Department of Urology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. cskim@amc.seoul.kr
- 2Department of Oncology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 1777555
- DOI: http://doi.org/10.3346/jkms.2013.28.5.687
Abstract
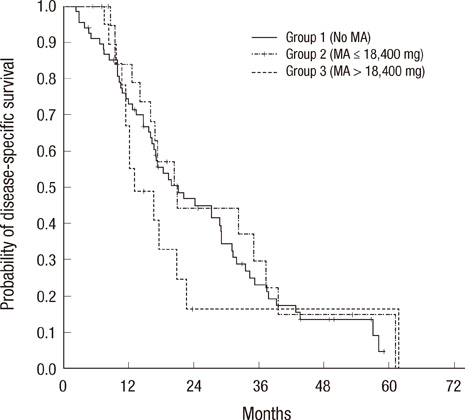
- There are concerns whether megestrol acetate (MA) stimulates the growth of prostate cancer in castration-resistant prostate cancer (CRPC). We evaluated the effect of cumulative doses of MA on the disease-specific survival (DSS) in patients with CRPC who were receiving Docetaxel-based chemotherapy. From July 2003 through June 2009, we identified 109 consecutive patients with CRPC and who had received docetaxel-based chemotherapy. Of these patients, 68 (62.4%) have not received MA, whereas 21 patients (19.3%) and 20 patients (18.3%) had received low dose MA (total < or = 18,400 mg) and high dose MA (total > 18,400 mg), respectively. We assessed the effect of several variables on DSS. None of the clinicopathological variables differed among the three groups. When comparing DSS using Kaplan-Meier analysis, there was no statistically significant survival differences among the three groups (P = 0.546). Using multivariate Cox proportional analyses with backward elimination, the number of docetaxel cycles was only significant factor predicting DSS (HR: 0.578, 95% CI: 0.318-0.923, P = 0.016). Cumulative doses of MA as adjuvant treatment for patients with CRPC and who are receiving docetaxel-based chemotherapy, did not affect their DSS. Therefore, MA can be safely administered in cachexic patients with CRPC.
MeSH Terms
-
Aged
Aged, 80 and over
Anorexia/complications/*drug therapy
Antineoplastic Agents/therapeutic use
Antineoplastic Agents, Hormonal/*therapeutic use
Cachexia/complications/*drug therapy
Castration
Humans
Kaplan-Meier Estimate
Male
Megestrol Acetate/*therapeutic use
Middle Aged
Proportional Hazards Models
Prostatic Neoplasms/complications/*drug therapy/mortality
Taxoids/therapeutic use
Antineoplastic Agents
Antineoplastic Agents, Hormonal
Taxoids
Megestrol Acetate
Figure
Reference
-
1. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012. 44:25–31.2. Chi BH, Chang IH. Prostate cancer: recent trends in Korea. Urol Int. 2010. 85:88–93.3. Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006. 24:3089–3094.4. Kim SJ, Kim SI. Current treatment strategies for castration-resistant prostate cancer. Korean J Urol. 2011. 52:157–165.5. Vogelzang NJ. One hundred thirteen men with hormone-refractory prostate cancer died today. J Clin Oncol. 1996. 14:1753–1755.6. Galsky MD, Vogelzang NJ. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann Oncol. 2010. 21:2135–2144.7. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004. 351:1502–1512.8. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004. 351:1513–1520.9. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients: Eastern Cooperative Oncology Group. Am J Med. 1980. 69:491–497.10. Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004. 114:370–378.11. Geller J, Albert J, Geller S, Lopez D, Cantor T, Yen S. Effect of megestrol acetate (Megace) on steroid metabolism and steroid-protein binding in the human prostate. J Clin Endocrinol Metab. 1976. 43:1000–1008.12. Geller J, Albert J, Yen SS. Treatment of advanced cancer of prostate with megestrol acetate. Urology. 1978. 12:537–541.13. Dawson NA, Conaway M, Halabi S, Winer EP, Small EJ, Lake D, Vogelzang NJ. A randomized study comparing standard versus moderately high dose megestrol acetate for patients with advanced prostate carcinoma: cancer and leukemia group B study 9181. Cancer. 2000. 88:825–834.14. Mateen F, Jatoi A. Megestrol acetate for the palliation of anorexia in advanced, incurable cancer patients. Clin Nutr. 2006. 25:711–715.15. Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome: the DATRI 004 Study Group: division of AIDS Treatment Research Initiative. AIDS Res Hum Retroviruses. 1997. 13:305–315.16. Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, Rowland KM Jr, Camoriano JK, Novotny PJ, Christensen BJ. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J Clin Oncol. 1999. 17:3299–3306.17. Jiang Y, Palma JF, Agus DB, Wang Y, Gross ME. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem. 2010. 56:1492–1495.18. Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Anti-androgens and the mutated androgen receptor of LNCaP cells: differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992. 31:2393–2399.19. Tassinari D, Fochessati F, Panzini I, Poggi B, Sartori S, Ravaioli A. Rapid progression of advanced "hormone-resistant" prostate cancer during palliative treatment with progestins for cancer cachexia. J Pain Symptom Manage. 2003. 25:481–484.20. Aus G, Abbou CC, Bolla M, Heidenreich A, Schmid HP, van Poppel H, Wolff J, Zattoni F. European Association of Urology. EAU guidelines on prostate cancer. Eur Urol. 2005. 48:546–551.21. Loprinzi CL, Michalak JC, Quella SK, O'Fallon JR, Hatfield AK, Nelimark RA, Dose AM, Fischer T, Johnson C, Klatt NE, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994. 331:347–352.22. Daniel F, MacLeod PM, Tyrrell CJ. Megestrol acetate in relapsed carcinoma of prostate. Br J Urol. 1990. 65:275–277.23. Patel SR, Kvols LK, Hahn RG, Windschitl H, Levitt R, Therneau T. A phase II randomized trial of megestrol acetate or dexamethasone in the treatment of hormonally refractory advanced carcinoma of the prostate. Cancer. 1990. 66:655–658.24. Dawson NA, McLeod DG. Dramatic prostate specific antigen decrease in response to discontinuation of megestrol acetate in advanced prostate cancer: expansion of the antiandrogen withdrawal syndrome. J Urol. 1995. 153:1946–1947.25. Sartor O, Eastham JA. Progressive prostate cancer associated with use of megestrol acetate administered for control of hot flashes. South Med J. 1999. 92:415–416.26. Loprinzi CL, Michalak JC, Schaid DJ, Mailliard JA, Athmann LM, Goldberg RM, Tschetter LK, Hatfield AK, Morton RF. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J Clin Oncol. 1993. 11:762–767.27. Bruera E, Macmillan K, Kuehn N, Hanson J, MacDonald RN. A controlled trial of megestrol acetate on appetite, caloric intake, nutritional status, and other symptoms in patients with advanced cancer. Cancer. 1990. 66:1279–1282.28. Kornblith AB, Hollis DR, Zuckerman E, Lyss AP, Canellos GP, Cooper MR, Herndon JE 2nd, Phillips CA, Abrams J, Aisner J, et al. Effect of megestrol acetate on quality of life in a dose-response trial in women with advanced breast cancer: the Cancer and Leukemia Group B. J Clin Oncol. 1993. 11:2081–2089.29. Bruera E. ABC of palliative care: anorexia, cachexia, and nutrition. BMJ. 1997. 315:1219–1222.30. Kim DI, Song JM, Chung HC. Clinical significance of free-to-total prostate-specific antigen (PSA) ratio in advanced prostate cancer patients with PSA less than 0.1 ng/ml after hormone treatment. Korean J Urol. 2012. 53:149–153.31. Ansari J, Hussain SA, Alhasso A, Mahmood R, Ansari A, Glaholm J. Role of second-line systemic treatment post-docetaxel in metastatic castrate resistant prostate cancer- current strategies and future directions. Anticancer Agents Med Chem. 2011. 11:296–306.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in Cancer Cachexia
- Secondary Adrenal Insufficiency Associated with Megestrol Acetate in a Patient with Lung Cancer
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Thrombosis in Patients with Acquired Immunodeficiency Syndrome