Yonsei Med J.
2011 May;52(3):495-501. 10.3349/ymj.2011.52.3.495.
Peroxisome Proliferator-Activated Receptor-Gamma Expression in the Lung Tissue of Obese Rats
- Affiliations
-
- 1Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. jy7.shim@samsung.com
- 2Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Research Institute of Medical Science, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1777023
- DOI: http://doi.org/10.3349/ymj.2011.52.3.495
Abstract
- PURPOSE
Obesity is a risk factor for asthma and type II diabetes. Peroxisome proliferator-activated receptor (PPAR)-gamma has been suggested to regulate inflammatory responses in diabetes and asthma. We investigated whether PPAR-alpha, PPAR-gamma, adiponectin receptors (AdipoR1, AdipoR2), leptin, and tumor necrosis factor (TNF)-alpha are expressed in rat lung tissues and whether the expression differs between obese Otsuka Long-Evans Tokushima Fatty (OLETF) and lean Long Evans Tokushima Otsuka (LETO) rats.
MATERIALS AND METHODS
Obese and lean rats were given with a high fat diet or a 30% restricted diet for 32 weeks, and their blood glucose levels and weights were monitored. After 32 weeks, mRNA levels of PPAR-alpha, PPAR-gamma, AdipoR1, AdipoR2, leptin, and TNF-alpha in lung tissues were measured using real time PCR.
RESULTS
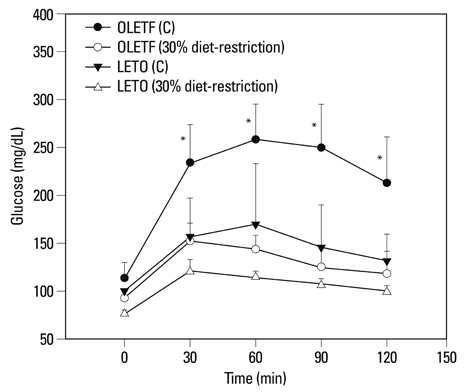
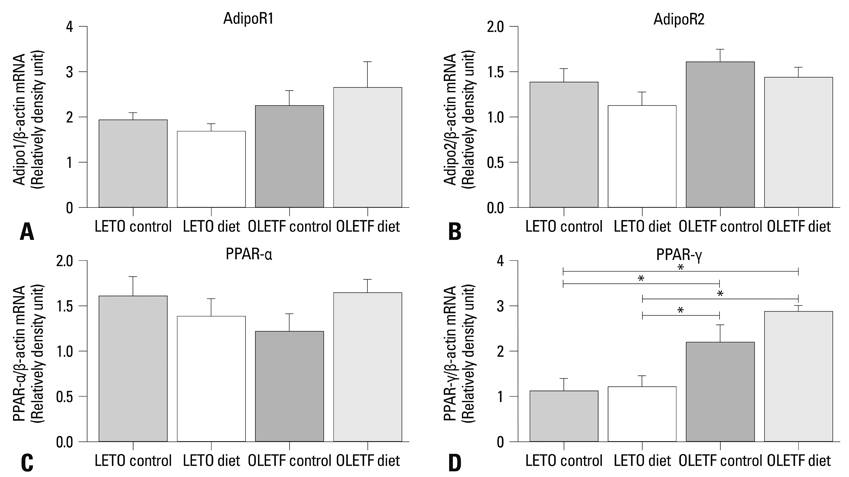
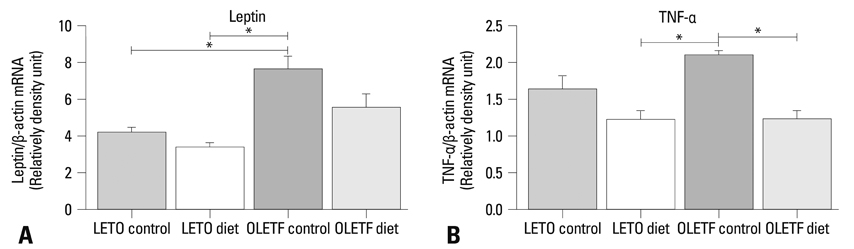
PPAR-alpha, PPAR-gamma, AdipoR1, AdipoR2, leptin, and TNF-alpha were expressed in both obese and lean rat lung tissues. Increased serum glucose levels on intraperitoneal glucose tolerance testing and a higher weight gain at 32 weeks were observed in OLETF control rats compared to OLETF diet restricted rats. PPAR-gamma expression was markedly elevated in obese control and diet restricted rats compared to lean rats, although PPAR-gamma expression in obese rats was not affected by diet restriction. Leptin was highly expressed in OLETF rats compared to LETO rats. TNF-alpha expression was enhanced in OLETF control rats compared LETO diet restricted rats, and decreased by diet restriction. PPAR-alpha, AdipoR1, and AdipoR2 expression were not significantly different between obese and lean rats.
CONCLUSION
PPAR-gamma was highly expressed in the lung tissues of obese rats and may be a novel treatment target for regulating lung inflammation associated with obesity.
Keyword
MeSH Terms
Figure
Reference
-
1. Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyperresponsiveness. J Allergy Clin Immunol. 2005. 115:925–927.
Article2. McClean KM, Kee F, Young IS, Elborn JS. Obesity and the lung: 1. Epidemiology. Thorax. 2008. 63:649–654.3. Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006. 174:112–119.
Article4. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005. 115:103–109.
Article5. Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003. 144:3765–3773.
Article6. Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003. 27:Suppl 3. S53–S55.
Article7. Nawrocki AR, Scherer PE. The delicate balance between fat and muscle: adipokines in metabolic disease and musculoskeletal inflammation. Curr Opin Pharmacol. 2004. 4:281–289.
Article8. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003. 112:1796–1808.
Article9. Elloumi M, Ben Ounis O, Makni E, Van Praagh E, Tabka Z, Lac G. Effect of individualized weight-loss programmes on adiponectin, leptin and resistin levels in obese adolescent boys. Acta Paediatr. 2009. 98:1487–1493.
Article10. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003. 52:1779–1785.11. Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003. 14:561–566.
Article12. Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988. 240:889–895.
Article13. Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000. 49:497–505.
Article14. Patel HJ, Belvisi MG, Bishop-Bailey D, Yacoub MH, Mitchell JA. Activation of peroxisome proliferator-activated receptors in human airway smooth muscle cells has a superior anti-inflammatory profile to corticosteroids: relevance for chronic obstructive pulmonary disease therapy. J Immunol. 2003. 170:2663–2669.
Article15. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998. 391:82–86.
Article16. Wang AC, Dai X, Luu B, Conrad DJ. Peroxisome proliferator-activated receptor-gamma regulates airway epithelial cell activation. Am J Respir Cell Mol Biol. 2001. 24:688–693.
Article17. Bishop-Bailey D, Warner TD. PPARgamma ligands induce prostaglandin production in vascular smooth muscle cells: indomethacin acts as a peroxisome proliferator-activated receptor-gamma antagonist. FASEB J. 2003. 17:1925–1927.18. Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998. 273:25573–25580.
Article19. British Thoraoic Society. British guideline on the management of asthma. Thorax. 2003. 58:Suppl 1. i1–i94.20. Barnes PJ. Inhaled glucocorticoids for asthma. N Engl J Med. 1995. 332:868–875.
Article21. Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003. 111:3–22.
Article22. Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005. 172:149–160.
Article23. Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994. 24:Suppl. S317–S320.
Article24. Belvisi MG, Hele DJ. Peroxisome proliferator-activated receptors as novel targets in lung disease. Chest. 2008. 134:152–157.
Article25. Faveeuw C, Fougeray S, Angeli V, Fontaine J, Chinetti G, Gosset P, et al. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-12 production in murine dendritic cells. FEBS Lett. 2000. 486:261–266.
Article26. Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B, et al. Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation. J Exp Med. 2003. 198:411–421.
Article27. Benayoun L, Letuve S, Druilhe A, Boczkowski J, Dombret MC, Mechighel P, et al. Regulation of peroxisome proliferator-activated receptor gamma expression in human asthmatic airways: relationship with proliferation, apoptosis, and airway remodeling. Am J Respir Crit Care Med. 2001. 164:1487–1494.
Article28. Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol. 2006. 533:101–109.
Article29. Honda K, Marquillies P, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptor gamma is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. J Allergy Clin Immunol. 2004. 113:882–888.
Article30. Hashimoto Y, Nakahara K. Improvement of asthma after administration of pioglitazone. Diabetes Care. 2002. 25:401.
Article31. Spears M, Donnelly I, Jolly L, Brannigan M, Ito K, McSharry C, et al. Bronchodilatory effect of the PPAR-gamma agonist rosiglitazone in smokers with asthma. Clin Pharmacol Ther. 2009. 86:49–53.
Article32. Kobayashi M, Thomassen MJ, Rambasek T, Bonfield TL, Raychaudhuri B, Malur A, et al. An inverse relationship between peroxisome proliferator-activated receptor gamma and allergic airway inflammation in an allergen challenge model. Ann Allergy Asthma Immunol. 2005. 95:468–473.
Article33. Xu B, Jarvelin MR, Pekkanen J. Body build and atopy. J Allergy Clin Immunol. 2000. 105:393–394.
Article34. Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999. 318:293–297.
Article35. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005. 115:911–919. quiz 920.
Article36. Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003. 112:1785–1788.
Article37. Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000. 199:15–24.
Article38. Shin JH, Kim JH, Lee WY, Shim JY. The expression of adiponectin receptors and the effects of adiponectin and leptin on airway smooth muscle cells. Yonsei Med J. 2008. 49:804–810.
Article39. Holguin F, Rojas M, Hart CM. The peroxisome proliferator activated receptor gamma (PPARgamma) ligand rosiglitazone modulates bronchoalveolar lavage levels of leptin, adiponectin, and inflammatory cytokines in lean and obese mice. Lung. 2007. 185:367–372.
Article40. Cabrero A, Cubero M, Llaverías G, Alegret M, Sánchez R, Laguna JC, et al. Leptin down-regulates peroxisome proliferator-activated receptor gamma (PPAR-gamma) mRNA levels in primary human monocyte-derived macrophages. Mol Cell Biochem. 2005. 275:173–179.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of peroxisome proliferator-activated receptor (PPAR)-alpha and PPAR-gamma in the lung tissue of obese mice and the effect of rosiglitazone on proinflammatory cytokine expressions in the lung tissue
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy
- Peroxisome Proliferator Activated Receptor-delta (PPAR-delta)
- Expression of the Peroxisome-proliferator-activated Receptor-gamma in Human Gastric Cancer
- Colorectal Cancer Expression of Peroxisome Proliferator-Activated Receptor gamma Is Associated with Good Prognosis