Yonsei Med J.
2005 Jun;46(3):353-358. 10.3349/ymj.2005.46.3.353.
The Involvement of Multipotential Progenitor Cells in Mooren's Ulcer
- Affiliations
-
- 1Department of Ophthalmology, Chung-Ang University College of Medicine, Seoul, Korea. jck50ey@kornet.net
- KMID: 1734069
- DOI: http://doi.org/10.3349/ymj.2005.46.3.353
Abstract
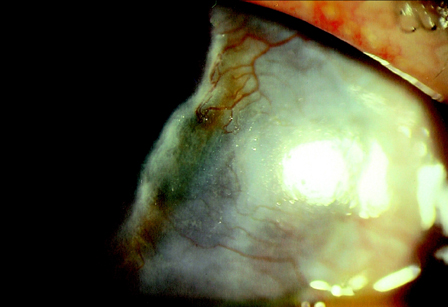
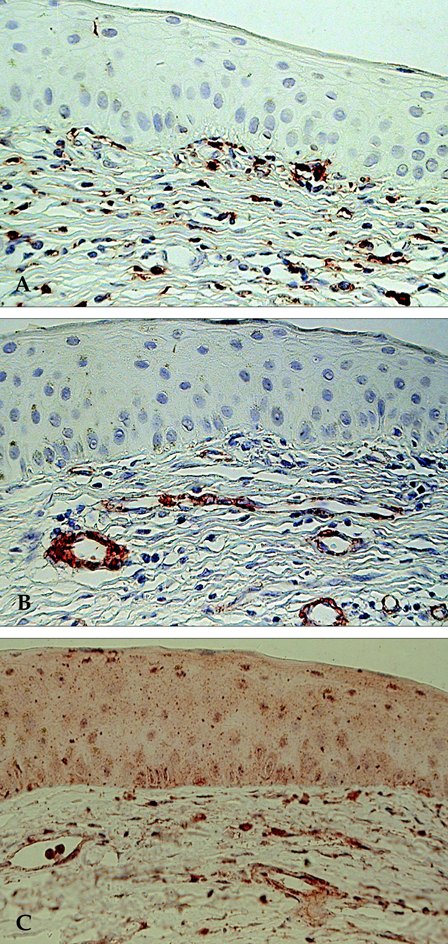
- The aim of this study was to assess the involvement of multipotential progenitor cells in the pathogenesis of Mooren's ulcer using immunohistochemical staining techniques. Tissue specimens were collected from 3 Mooren's ulcer patients who underwent lamellar keratectomy. Immunohistochemical staining patterns were analyzed using antibodies: CD34, c-kit, STRO-1, CD45RO, VEGF and alpha-SMA. Strong positive CD34, c-kit and STRO-1 cells were revealed in Mooren's ulcer specimens, especially in the superficial stroma. A few weakly expressed CD34 stroma cells were seen in normal limbal cornea but no immunoreactivity for c-kit and STRO-1 could be found. CD45RO positive T cells were found to have infiltrated in Mooren's ulcer. The immunostaining pattern of VEGF and yen a- SMA was closely correlated with the degree of expression and the number of CD34 positive cells. Bone marrow-derived multipotential progenitor cells may be involved in the pathogenesis of Mooren's ulcer by synergizing with other factors to amplify autoimmune destructive reactions and to contribute to the regeneration process. Specific therapeutic strategies that target the role of these cells in the disease are warranted.
MeSH Terms
Figure
Reference
-
1. Robin JB, Schanzlin DJ, Verity SM, Barron BA, Arffa RC, Suarez E, et al. Peripheral corneal disorders. Surv Ophthalmol. 1986. 31:1–36.2. Brown SI. What is Mooren's ulcer? Trans Ophthalmol Soc UK. 1978. 98:390–392.3. Wood TO, Kaufman HE. Mooren's ulcer. Am J Ophthalmol. 1971. 71:417–422.4. Tabbara KF. Mooren's ulcer. Int Ophthalmol Clin. 1986. 26:91–98.5. Young RD, Watson PG. Light and electron microscopy of corneal melting syndrome (Mooren's ulcer). Br J Ophthalmol. 1982. 66:341–356.6. Gottsch JD, Liu SH, Minkovitz JB, Goodman DF, Srinivasan M, Stark WJ. Autoimmunity to a cornea-associated stroma antigen in patients with Mooren's ulcer. Invest Ophthalmol Vis Sci. 1995. 36:1541–1547.7. Eiferman RA, Hyndiuk RA, Hensley GT. Limbal immunopathology of Mooren's ulcer. Ann Ophthalmol. 1978. 10:1203–1206.8. Lopez JS, Price FW, Whitcup SM, Li Q, de Smet M, Chen CC, et al. Immunopathology of Mooren's ulcer. Arch Ophthalmol. 1991. 109:988–992.9. Zhao JC, Jin XY. Immunological analysis and treatment of Mooren's ulcer with cyclosporin A. Cornea. 1993. 12:481–488.10. Romagnani S. Th1 and Th2 in human disese. Clin Immunol Immunopathol. 1996. 80:225–235.11. Giscombe R, Nityanand S, Lewin N, Grunewald J, Lefvert AK. Expanded T cell populations in patients with Wegener's granulomatosis: characteristic and correlates with disease activity. J Clin Immunol. 1998. 18:404–413.12. Ludviksson BR, Sneller MC, Chua KS, Talar-Williams C, Langford CA, Ehrhardt RO, et al. Active Wegener's granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998. 160:3602–3609.13. John SL, Morgan K, Tullo AB, Holt PJ. Corneal autoimmunity in patients with peripheral ulcerative keratitis (PUK) in association with rheumatoid arthritis and Wegener's granulomatosis. Eye. 1992. 6:630–636.14. Risau W. Differentiation of endothelium. FASEB J. 1995. 9:926–933.15. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. 275:964–967.16. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–228.17. Boyd AW. Human leukocyte antigens: an update on structure, function and nomenclature. Pathology. 1987. 19:329–337.18. Watt SM, Karhi K, Gatter K, Furley AJ, Katz FE, Healy LE, et al. Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human progenitor cells. Leukaemia. 1987. 1:417–426.19. Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, et al. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990. 62:690–696.20. Dennis JE, Carbillet JP, Caplan AI, Charbord P. The STRO-1+ marrow cell population is multipotential. Cell Tissues Organs. 2002. 170:73–82.21. Huss R, Moosmann S. The co-expression of CD117 (c-kit) and osteocalcin in activated bone marrow stem cells in different disease. Br J Haematol. 2002. 118:305–312.22. Chow CY, Foster CS. Mooren's ulcer. Int Ophthalmol Clin. 1996. 36:1–13.23. Frangiegh T, Kenyon KR. Brightbill FS, editor. Mooren's ulcer. Corneal surgery: theory, technique and tissue. 1993. ed2. St Louis: Mosby.24. Robin JB, Dugal R. Kaufman HE, Barron BA, McDonald MB, Saltman SR, editors. Immunologic disorders of the cornea and conjunctiva. The Cornea. 1988. New York: Churchill Livingstones.25. Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001. 103:2776–2779.26. Risau W, Flamme I. Vasculogenesis. Anna Rev Cell Biol. 1995. 11:73–91.27. Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against α-smooth muscle differentiation. J Cell Biol. 1986. 103:2787–2796.28. Peault BM, Thiery JP, LeDouarin NM. Surface marker for hemopoietic and sndothelial cell lineages that is defined by monoclonal antibody. Pro Natl Acad Sci USA. 1983. 80:2976–2980.29. Garlanda C, Dejana E. Heterogeneity of endothelial cells: specific markers. Arterioscler Thromb Vasc Biol. 1997. 17:1193–1202.30. Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990. 63:213–224.31. Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990. 63:225–233.32. Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990. 63:235–243.33. Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994. 84:4164–4173.34. Simmons PJ, Torok SB. Identification of stroma cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991. 78:55–62.35. Mondino BJ. Inflammatory disease of the peripheral cornea. Ophthalmology. 1988. 95:463–472.36. Mondino BJ. Experimental aspects and models of peripheral corneal disease. Int Ophthalmol Clin. 1986. 26:5–14.37. Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. 2000. 19:625–643.38. Gregory JK, Foster CS. Peripheral ulcerative keratitis in the collagen vascular disease. Int Ophthalmol Clin. 1996. 36:21–30.39. Shiuey Y, Foster CS. Peripheral ulcerative keratitis and collagen vascular disease. Int Ophthalmol Clin. 1998. 38:21–32.40. Gottsch JD, Li Q, Ashraf F, O'Brien TP, Stark WJ, Liu SH. Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol. 1999. 91:34–40.41. Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000. 9:841–848.42. Marti T, Ertmann KD, Gallin MY. Host-parasite interaction in human oncocherciasis: identification and sequence analysis of novel human calgranulin. Biochem Biophys Res Commun. 1996. 221:454–458.