Clin Exp Vaccine Res.
2014 Jul;3(2):185-193. 10.7774/cevr.2014.3.2.185.
Cellular immunity survey against urinary tract infection using pVAX/fimH cassette with mammalian and wild type codon usage as a DNA vaccine
- Affiliations
-
- 1Applied Microbiology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran. imanifouladi.a@gmail.com
- 2Bacteriology Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
- 3Department of Genetic Engineering, Faculty of Biosciences and Biotechnology, Malekashtar University of Technology, Tehran, Iran.
- 4Department of Virology, Pasteur Institute of Iran, Tehran, Iran.
- 5Department of Drug and Food Control, Tehran University of Medical Sciences, Tehran, Iran.
- 6Department of Physiology and Pharmacology, Pasteur Institute of Iran, Tehran, Iran.
- 7Nephrology and Urology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
- KMID: 1730623
- DOI: http://doi.org/10.7774/cevr.2014.3.2.185
Abstract
- PURPOSE
FimH (the adhesion fragment of type 1 fimbriae) is implicated in uropathogenic Escherichia coli (UPEC) attachment to epithelial cells through interaction with mannose. Recently, some studies have found that UPEC can thrive intracellularly causing recurrent urinary tract infection (UTI). Almost all vaccines have been designed to induce antibodies against UPEC. Yet, the humoral immune response is not potent enough to overcome neither the primary UTI nor recurrent infections. However, DNA vaccines offer the possibility of inducing cell mediated immune responses and may be a promising preventive tool.
MATERIALS AND METHODS
In this study, we employed two different open reading frames within mammalian (mam) and wild type (wt) codons of fimH gene. Optimized fragments were cloned in pVAX-1. Expression of the protein in COS-7 was confirmed by western blot analysis after assessing pVAX/fimH(mam) and pVAX/fimH(wt). The constructs were injected to BALB/c mice at plantar surface of feet followed by electroporation.
RESULTS
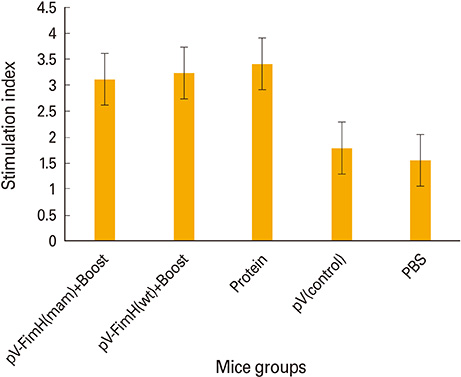
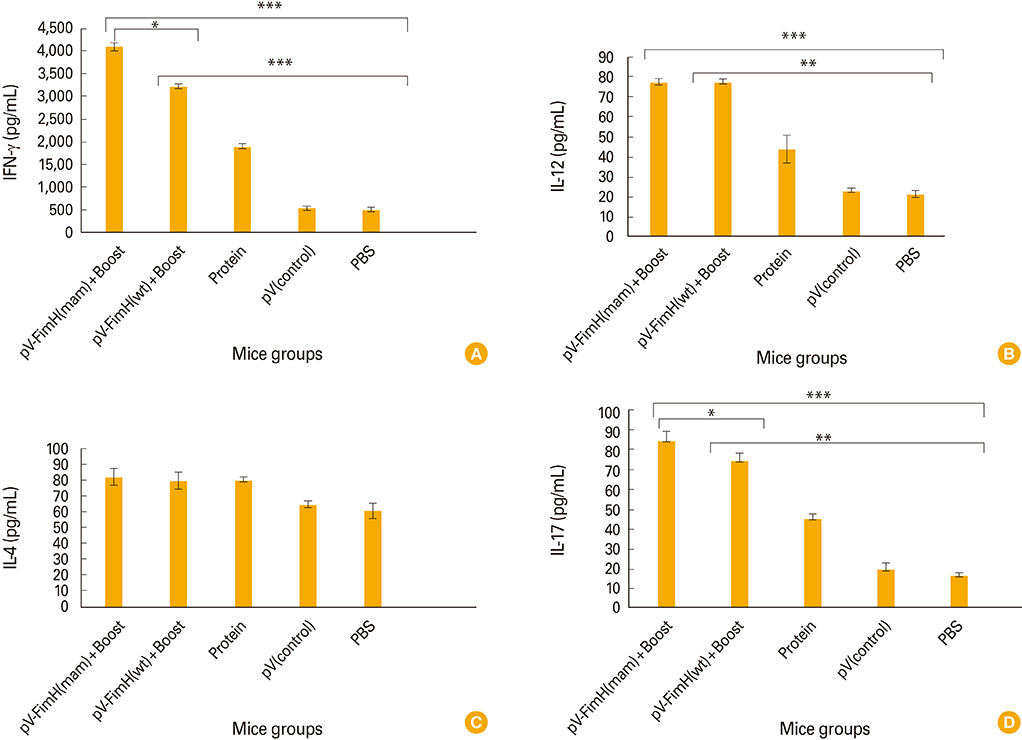
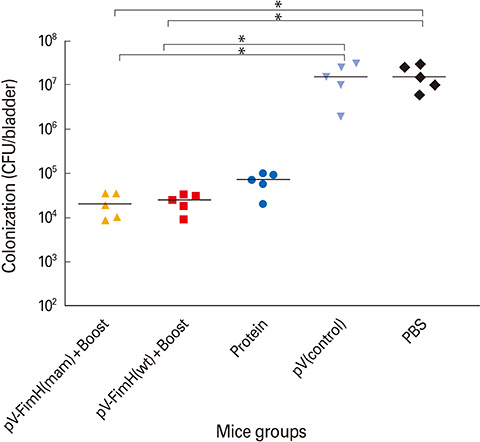
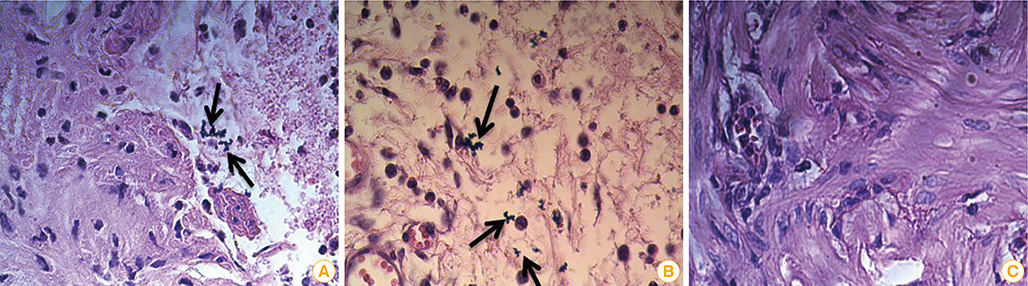
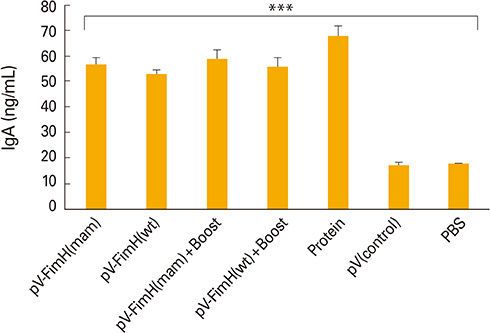
The mice immunized with both constructs following booster injection with recombinant FimH showed increased interferon-gamma and interleukin-12 responses significantly higher than non-immunized ones (p<0.05). The immunized mice were challenged with UPEC and then the number of bacteria recovered from the immunized mice was compared with the non-immunized ones. Decreased colony count in immunized mice along with cytokine responses confirmed the promising immune response by the DNA vaccines developed in this study.
CONCLUSION
In conclusion, DNA vaccines of UPEC proteins may confer some levels of protection which can be improved by multiple constructs or boosters.
MeSH Terms
-
Animals
Antibodies
Bacteria
Blotting, Western
Clone Cells
Codon*
DNA*
Electroporation
Epithelial Cells
Foot
Immunity, Cellular*
Immunity, Humoral
Interferon-gamma
Interleukin-12
Mannose
Mice
Open Reading Frames
Urinary Tract Infections*
Uropathogenic Escherichia coli
Vaccines
Vaccines, DNA
Antibodies
Codon
DNA
Interferon-gamma
Interleukin-12
Mannose
Vaccines
Vaccines, DNA
Figure
Reference
-
1. Klumpp DJ, Rycyk MT, Chen MC, Thumbikat P, Sengupta S, Schaeffer AJ. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect Immun. 2006; 74:5106–5113.
Article2. Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem. 2002; 277:7412–7419.
Article3. Blomgran R, Zheng L, Stendahl O. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect Immun. 2004; 72:4570–4578.
Article4. Wright KJ, Seed PC, Hultgren SJ. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol. 2007; 9:2230–2241.
Article5. Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998; 282:1494–1497.
Article6. Cirl C, Wieser A, Yadav M, et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat Med. 2008; 14:399–406.
Article7. Billips KA, Schaeffer J, Klumpp DJ. Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder. Infect Immun. 2008; 76:3891–3900.
Article8. Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun. 2005; 73:3999–4006.
Article9. Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000; 97:8829–8835.
Article10. Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun. 2010; 78:568–585.
Article11. Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011; 53:296–302.
Article12. Spearman P, Kalams S, Elizaga M, et al. Safety and immunogenicity of a CTL multiepitope peptide vaccine for HIV with or without GM-CSF in a phase I trial. Vaccine. 2009; 27:243–249.
Article13. Kutzler MA, Weiner DB. Developing DNA vaccines that call to dendritic cells. J Clin Invest. 2004; 114:1241–1244.
Article14. Bagherpour G, Fooladi AA, Mehrabadi JF, Nourani MR, Einollahi B. Evaluation of mammalian codon usage of fimH in DNA vaccine design. Acta Microbiol Immunol Hung. 2011; 58:259–271.
Article15. Johnson DE, Lockatell CV. Mouse model of ascending UTI involving short- and long-term indwelling catheters. In : Zak O, Sande MA, editors. Handbook of animal models of infection. Experimental models in antimicrobial chemotherapy. London: Academic Press;1999. p. 441–445.16. Kim OY, Hong BS, Park KS, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2013; 190:4092–4102.
Article17. Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001; 69:4572–4579.
Article18. Schilling JD, Lorenz RG, Hultgren SJ. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect Immun. 2002; 70:7042–7049.
Article19. Goluszko P, Goluszko E, Nowicki B, Nowicki S, Popov V, Wang HQ. Vaccination with purified Dr Fimbriae reduces mortality associated with chronic urinary tract infection due to Escherichia coli bearing Dr adhesin. Infect Immun. 2005; 73:627–631.
Article20. O'Hanley P, Lark D, Falkow S, Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985; 75:347–360.21. Poggio TV, La Torre JL, Scodeller EA. Intranasal immunization with a recombinant truncated FimH adhesin adjuvanted with CpG oligodeoxynucleotides protects mice against uropathogenic Escherichia coli challenge. Can J Microbiol. 2006; 52:1093–1102.
Article22. Langermann S, Mollby R, Burlein JE, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis. 2000; 181:774–778.
Article23. Supek F, Smuc T. On relevance of codon usage to expression of synthetic and natural genes in Escherichia coli. Genetics. 2010; 185:1129–1134.
Article24. Ko HJ, Ko SY, Kim YJ, Lee EG, Cho SN, Kang CY. Optimization of codon usage enhances the immunogenicity of a DNA vaccine encoding mycobacterial antigen Ag85B. Infect Immun. 2005; 73:5666–5674.
Article25. Tokuoka M, Tanaka M, Ono K, Takagi S, Shintani T, Gomi K. Codon optimization increases steady-state mRNA levels in Aspergillus oryzae heterologous gene expression. Appl Environ Microbiol. 2008; 74:6538–6546.
Article26. Uchijima M, Yoshida A, Nagata T, Koide Y. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J Immunol. 1998; 161:5594–5599.27. Burgess-Brown NA, Sharma S, Sobott F, Loenarz C, Oppermann U, Gileadi O. Codon optimization can improve expression of human genes in Escherichia coli: A multi-gene study. Protein Expr Purif. 2008; 59:94–102.
Article28. Wang Q, Mei C, Zhen H, Zhu J. Codon preference optimization increases prokaryotic cystatin C expression. J Biomed Biotechnol. 2012; 2012:732017.
Article29. Asadi Karam MR, Oloomi M, Mahdavi M, Habibi M, Bouzari S. Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine. 2013; 31:1210–1216.
Article30. Titomirov AV, Sukharev S, Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991; 1088:131–134.
Article31. Rosati M, Valentin A, Jalah R, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008; 26:5223–5229.
Article32. Crottet P, Corthesy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab')2: a possible implication for mucosal defense. J Immunol. 1998; 161:5445–5453.33. Ma JK, Hikmat BY, Wycoff K, et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998; 4:601–606.
Article34. Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. J Med Microbiol. 1998; 47:879–888.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Immunity Induced at Priming Step on Mucosal Immunization of Heterologous Prime-Boost Regimens
- Recombinant DNA and Protein Vaccines for Foot-and-mouth Disease Induce Humoral and Cellular Immune Responses in Mice
- Phylogenetic Groups and Virulence Factors of Escherichia coli Causing Urinary Tract Infection in Children
- cGAS-cGAMP Signaling and Antiviral Defense
- Clinical Significance of Toll-Like Receptor and Toll-Like Receptor Blocker