Yonsei Med J.
2006 Jun;47(3):399-404. 10.3349/ymj.2006.47.3.399.
Simplified EM Grid Vitrification Is a Convenient and Efficient Method for Mouse Mature Oocyte Cryopreservation
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Seoul National University, Seoul, Korea. shmoon@plaza.snu.ac.kr
- 2Institute of Reproductive Medicine and Population, Medical Research Center, Seoul National University, Seoul, Korea.
- KMID: 1715860
- DOI: http://doi.org/10.3349/ymj.2006.47.3.399
Abstract
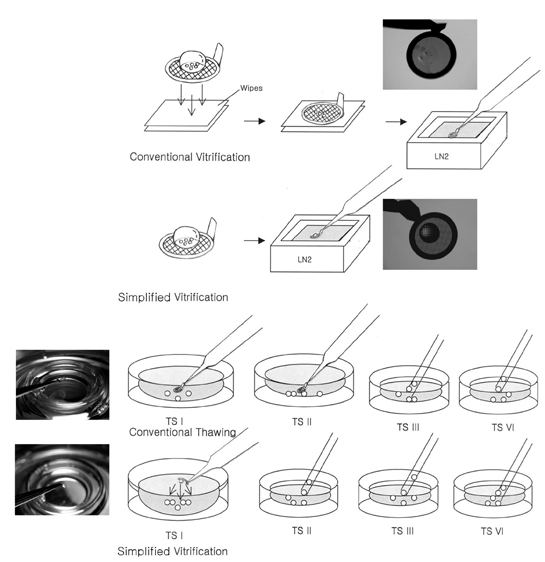
- This study was performed to evaluate the efficiency of simplified EM grid vitrification, skipping the step of removing the cryoprotectant (5.5M EG + 1.0M sucrose) droplet on the grid after loading oocytes, compared to conventional cryopreservation protocols for mouse mature oocytes. Firstly, the recovery, survival, fertilization and hatching rates of simplified EM grid vitrification were compared with those of the slow freezing method using 1.5M DMSO. Then, conventional EM grid vitrification was compared with simplified EM grid vitrification. Simplified EM grid vitrification showed higher survival, fertilization and hatching rates than those of the slow freezing method (85.6% vs. 63.2%; 51.0% vs. 22.3%; 38.7% vs. 12.5%, p < 0.01, respectively). Moreover, simplified EM grid vitrification showed higher recovery, survival and fertilization rates than those of conventional EM grid vitrification (100% vs. 95.0%, p=0.024; 90.0% vs. 78.9%, p=0.033; 56.7% vs. 38.7%, p=0.021, respectively). Hatching rate tended to be higher for simplified EM grid vitrification compared to conventional EM grid vitrification (41.1% vs. 24.1%). In conclusion, simplified EM grid vitrification is a convenient and efficient method for cryopreservation of mouse mature oocytes, compared to conventional EM grid vitrification and slow freezing methods.
MeSH Terms
Figure
Reference
-
1. Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986. 1:884–886.2. Van Uem JF, Siebzehnrubl ER, Schuh B, Koch R, Trotnow S, Lang N. Birth after cryopreservation of unfertilized oocytes. Lancet. 1987. 1:752–753.3. Porcu E, Fabbri R, Seracchioli R, Ciotti PM, Magrini O, Flamigni C. Birth of a healthy female after intracytoplasmic sperm injection of cryopreserved human oocytes. Fertil Steril. 1997. 68:724–726.4. Gook DA, Osbom SM, Bourne H, Johnston WI. Fertilization of human oocytes following cryopreservation: normal karyotypes and absence of stary chromosomes. Hum Reprod. 1994. 9:684–691.5. Tucker M, Wright G, Morton P, Shanguo L, Massey J, Kort H. Preliminary experience with human oocyte cryopreservation using 1,2-propanediol and sucrose. Hum Reprod. 1996. 11:1513–1515.6. Matson PL, Graefling J, Junk SM, Yovich JL, Edirisinghe WR. Cryopreservation of oocytes and embryos: use of a mouse model to investigate effects upon zona hardness and formulate treatment strategies in an in-vitro fertilization programme. Hum Reprod. 1997. 12:1550–1553.7. Mandelbaum J, Belaisch-Allart J, Junca AM, Antoine JM, Plachot M, Alvarez S, et al. Cryopreservation in human assisted reproduction is now routine for embryos but remains a research procedure for oocytes. Hum Reprod. 1998. 13:Suppl 3. 161–177.8. Fabbri R, Porcu E, Marsella T, Primavera MR, Rocchetta G, Ciotti PM, et al. Technical aspects of oocyte cryopreservation. Mol Cell Endocrinol. 2000. 169:39–42.9. Park SE, Chung HM, Cha KY, Hwang WS, Lee ES, Lim JM. Cryopreservation of ICR mouse oocytes: improved post-thawed preimplantation development after vitrification using Taxol, a cytoskeleton stabilizer. Fertil Steril. 2001. 75:1177–1184.10. Van der Elst J. Oocyte freezing: here to stay? Hum Reprod Update. 2003. 9:463–470.11. Whittingham DG. Fertilization in vitro and development to term of unfertilized mouse oocytes previously stored at -196 degrees C. J Reprod Fertil. 1977. 49:89–94.12. Vincent C, Pickering SJ, Johnson MH. The hardening effect of dimethylsulphoxide on the mouse zona pellucida requires the presence of an oocyte and is associated with a reduction in the number of cortical granules present. J Reprod Fertil. 1990. 89:253–259.13. Wood MJ, Whittingham DG, Lee SH. Fertilization failure of frozen mouse oocytes is not due to premature cortical granule release. Biol Reprod. 1992. 46:1187–1195.14. Bouquet M, Selva J, Auroux M. Effects of cooling and equilibration in DMSO, and cryopreservation of mouse oocytes, on the rates of in vitro fertilization, development, and chromosomal abnormalities. Mol Reprod Dev. 1995. 40:110–115.15. Kola I, Kirby C, Shaw J, Davey A, Trounson A. Vitrification of mouse oocytes results in aneuploid zygotes and malformed fetuses. Teratology. 1988. 38:467–474.16. Ruppert-Lingham CJ, Paynter SJ, Godfrey J, Fuller BJ, Shaw RW. Developmental potential of murine germinal vesicle stage cumulus-oocyte complexes following exposure to dimethylsulphoxide or cryopreservation: loss of membrane integrity of cumulus cells after thawing. Hum Reprod. 2003. 18:392–398.17. Paynter SJ, Fuller BJ, Shaw RW. Temperature dependence of mature mouse oocyte membrane permeabilities in the presence of cryoprotectant. Cryobiology. 1997. 34:122–130.18. Rayos AA, Takahashi Y, Hishinuma M, Kanagawa H. Quick freezing of unfertilized mouse oocytes using ethylene glycol with sucrose or trehalose. J Reprod Fertil. 1994. 100:123–129.19. O'Neil L, Paynter SJ, Fuller BJ, Shaw RW, DeVries AL. Vitrification of mature mouse oocytes in a 6 M Me2SO solution supplemented with antifreeze glycoproteins: the effect of temperature. Cryobiology. 1998. 37:59–66.20. Lane M, Gardner DK. Vitrification of mouse oocytes using a nylon loop. Mol Reprod Dev. 2001. 58:342–347.21. Mavrides A, Morroll D. Cryopreservation of bovine oocytes: is cryoloop vitrification the future to preserving the female gamete? Reprod Nutr Dev. 2002. 42:73–80.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Effective Cryopreservation Method for Mouse Oocytes
- Effect of Cytochalasin B on Survivability and in Vitro Development of Mouse Oocyte Frozen by Vitrification
- In vitro Fertilization and Embryo Development in Simple Media of the Frozen-Thawed Cumulus-free Mouse Oocytes Cryopreserved by Vitrification
- The Effect of Cytochalasin B on Cytoskeletal Stability of Mouse Oocyte Frozen by Vitrification
- Study on the Development of Efficient Vitrification of Human Blastocysts