Yonsei Med J.
2006 Jun;47(3):293-306. 10.3349/ymj.2006.47.3.293.
An Update of the Defensive Barrier Function of Skin
- Affiliations
-
- 1Department of Dermatology, Yonsei University College of Medicine, Seoul, Korea. ydshderm@yumc.yonsei.ac.kr
- 2Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Dermatology, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 1715846
- DOI: http://doi.org/10.3349/ymj.2006.47.3.293
Abstract
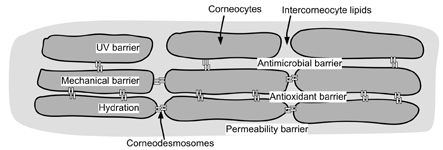
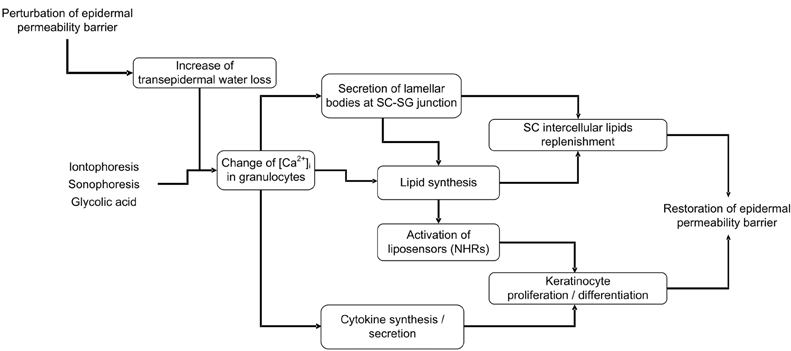
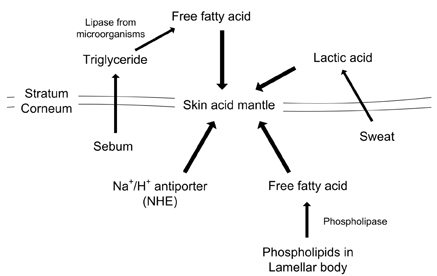
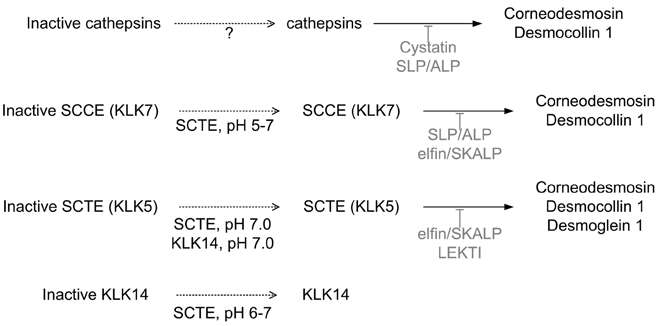
- Skin, as the outermost organ in the human body, continuously confronts the external environment and serves as a primary defense system. The protective functions of skin include UV-protection, anti-oxidant and antimicrobial functions. In addition to these protections, skin also acts as a sensory organ and the primary regulator of body temperature. Within these important functions, the epidermal permeability barrier, which controls the transcutaneous movement of water and other electrolytes, is probably the most important. This permeability barrier resides in the stratum corneum, a resilient layer composed of corneocytes and stratum corneum intercellular lipids. Since the first realization of the structural and biochemical diversities involved in the stratum corneum, a tremendous amount of work has been performed to elucidate its roles and functions in the skin, and in humans in general. The perturbation of the epidermal permeability barrier, previously speculated to be just a symptom involved in skin diseases, is currently considered to be a primary pathophysiologic factor for many skin diseases. In addition, much of the evidence provides support for the idea that various protective functions in the skin are closely related or even co-regulated. In this review, the recent achievements of skin researchers focusing on the functions of the epidermal permeability barrier and their importance in skin disease, such as atopic dermatitis and psoriasis, are introduced.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Atopic dermatitis and skin barrier dysfunction
Hyunjung Kim, Jung U Shin, Kwang Hoon Lee
Allergy Asthma Respir Dis. 2013;1(1):20-28. doi: 10.4168/aard.2013.1.1.20.
Reference
-
1. Albanesi C, Scarponi C, Giustizieri ML, Girolomoni G. Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005. 4:329–334.2. Hearing VJ. Biogenensis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005. 37:3–14.3. Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004. 13:Suppl 4. 11–15.4. Garssen J, Vandebriel RJ, van Loveren H. Molecular aspects of UVB-induced immunosuppression. Arch Toxicol Suppl. 1997. 19:97–109.5. Ghoreishi M. Heat shock proteins in the pathogenesis of inflammatory skin diseases. J Med Dent Sci. 2000. 47:143–150.6. Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defense peptides in mammalian immunity. Curr Protein Pept Sci. 2005. 6:35–51.7. Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005. 125:9–13.8. Elias PM. Epidermal lipids, barrier function and desquamation. J Invest Dermatol. 1983. 80:Suppl. 44–49.9. Jarnik M, Simon MN, Steven AC. Cornified cell envelope assembly: a model based on electron microscopic determinations of thickness and projected density. J Cell Sci. 1998. 111:1051–1060.10. Swartzendruber DC, Wertz PW, Madison KC, Downing DT. Evidence that the corneocytes has a chemically bound lipid envelope. J Invest Dermatol. 1987. 88:709–713.11. Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays. 2002. 24:789–800.12. Komatsu N, Saijoh K, Sidiropoulos M, Tsai B, Levesque MA, Elliott MB, et al. Quantification of human tissue kallikreins in the stratum corneum: dependence on age and gender. J Invest Dermatol. 2005. 125:1182–1189.13. Elias PM, Crumrine D, Rassner U, Hachem JP, Menon GK, Man W, et al. Basis for abnormal desquamation and permeability barrier dysfunction in RXLI. J Invest Dermatol. 2004. 122:314–319.14. Schechter NM, Choi EJ, Wang ZM, Hanakawa Y, Stanley JR, Kang Y, et al. Inhibition of human kallikreins 5 and 7 by the serine protease inhibitor lymphoepithelial Kazal-type inhibitor (LEKTI). Biol Chem. 2005. 386:1173–1184.15. Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, β-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003. 74:180–182.16. Braff MH, Di Nardo A, Gallo RL. Keratinocytes stores the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005. 124:394–400.17. Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antimicrobial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997. 272:15258–15263.18. Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003. 112:1195–1202.19. de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005. 125:1163–1173.20. Kim M, Jeon H, Goo J, Ahn S, Park Y, Lee S, et al. Expression of anti-microbial peptides in murine epidermis in increased by UVB exposure. J Invest Dermatol. 2006. 126:A66.21. Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci. 2004. 117:131–132.22. Papp H, Czifra G, Lazar J, Gonczi M, Csernoch L, Kovacs L, et al. Protein kinase C isozymes regulate proliferation and high cell density-mediated differentiation in HaCaT keratinocytes. Exp Dermatol. 2003. 12:811–824.23. Murata S, Uchida Y, Ichikawa S, Hirabayashi Y, Holleran WM. Regulation of glucosylceramide synthase expression in cultured human keratinocyte. J Invest Dermatol. 2000. 114:795.24. Ahn BK, Jeong SK, Kim HS, Choi KJ, Seo JT, Choi EH, et al. Rottlerin, a specific inhibitor of protein kinase C-delta, impedes barrier repair responses by increasing intracellular free calcium. J Invest Dermatol. 2006. 126:1348–1355.25. Odland GF, Holbrook A. The lamellar granules of epidermis. Curr Probl Derm. 1981. 9:29–49.26. Menon GK, Ghadially R, Williams ML, Elias PM. Lamellar bodies as delivery systems of hydrolytic enzymes: implications for normal and abnormal desquamation. Br J Dermatol. 1992. 126:337–345.27. Ishida-Yamamoto A, Simon M, Kishibe M, Miyauchi Y, Takahashi H, Yoshida S, et al. Epidermal lamellar granules transport different cargoes as distinct aggregates. J Invest Dermatol. 2004. 122:1137–1144.28. Ishida-Yamamoto A, Deraison C, Bonnart C, Bitoun E, Robinson R, O'Brien TJ, et al. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005. 124:360–366.29. Madison KC, Howard EJ. Ceramides are transported through the Golgi apparatus in human keratinocytes in vitro. J Invest Dermatol. 1996. 106:1030–1035.30. Sando GN, Zhu H, Wies JM, Richman JT, Wertz PW, Madison KC. Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Invest Dermatol. 2003. 120:531–541.31. Elias PM, Cullander C, Mauro T, Rassner U, Komuves L, Brown BE, et al. The secretory granular cell: The outermost granular cells as a specialized secretory cells. J Investig Dermatol Symp Proc. 1998. 3:87–100.32. Guest AFG, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signalingm and disease. Biochem Cell Biol. 2004. 82:129–144.33. Menon GK. Caveolins in epidermal lamellar bodies: skin is an interactive interface, not an inflexible barrier. J Invest Dermatol. 2003. 120:15–16.34. Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, et al. Absence of caveolin-1 sensitized mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol. 2003. 162:2029–2039.35. Reolandt T, Huth M, Houben E, Crumrine D, Kutsumo A, Giddelo G, et al. Caveolin-1 incorporation into lipid rafts in the outer stratum granulosum suppresses lamellar body secretion in parallel with initiation of terminal differentiation. J Invest Dermatol. 2006. 126:A71.36. Park BD, Youm JK, Jeong SK, Choi EH, Ahn SK, Lee SH. The characterization of molecular organization of multilmellar emulsions containing pseudoceramide and type III synthetic ceramide. J Invest Dermatol. 2003. 121:794–801.37. Elias PM. Epidermal barrier function: intercellular lamellar lipid structures, origin, composition and metabolism. J Control Release. 1991. 15:199–262.38. Bouwstra JA, Gooris GS, Weerheim A, Ponec M. pH, cholesterol sulfate and fatty acids affects the stratum corneum lipid organization. J Invest Dermatol Symp Proc. 1998. 3:69–74.39. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002. 53:409–435.40. Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands for peroxisome proliferators-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997. 11:779–791.41. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005. 125:183–200.42. Man M-Q, Choi EH, Schmuth M, Crumrine D, Uchida Y, Elias PM, et al. Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: liposensors stimulate lipid synthesis, lamellar body secretion, and post-secretory lipid processing. J Invest Dermatol. 2006. 126:386–392.43. Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985. 84:508–512.44. Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992. 89:530–538.45. Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Rep. 1992. 270:503–512.46. Elias PM, Ahn SK, Brown BE, Crumrine D, Feingold KR. Origin of epidermal calcium gradient: regulation of barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002. 119:1269–1274.47. Menon GK, Price LF, Bommannan B, Elias PM, Feingold KR. Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion. J Invest Dermatol. 1994. 102:789–795.48. Lee SH, Choi EH, Feingold KR, Jiang S, Ahn SK. Iontophoresis itself on hairless mouse skin induces the loss of the epidermal calcium gradient without skin barrier impairment. J Invest Dermatol. 1998. 111:39–43.49. Choi EH, Kim MJ, Yeh BI, Ahn SK, Lee SH. Iontophoresis and sonophoresis stimulate epidermal cytokine expression at energies that do not provoke a barrier abnormality: lamellar body secretion and cytokine expression are linked to altered epidermal calcium levels. J Invest Dermatol. 2003. 121:1138–1144.50. Denda M, Inoue K, Inomata S, Denda S. γ-aminobutyric acid (A) receptor agonists accelerate cutaneous barrier recovery and prevent epidermal hyperplasia induced by barrier disruption. J Invest Dermatol. 2002. 119:1041–1047.51. Denda M, Inoue K, Fuziwara S, Denda S. P2X purinergic receptor antagonist accelerates skin barrier repair and prevents epidermal hyperplasia induced by skin barrier disruption. J Invest Dermatol. 2002. 119:1034–1040.52. Fuziwara S, Inoue K, Denda M. NMDA-type glutamate receptor is associated with cutaneous barrier homeostasis. J Invest Dermatol. 2003. 120:1023–1029.53. Denda M, Fuziwara S, Inoue K. β2-adrenergic receptor antagonist accelerates skin barrier recovery and reduces epidermal hyperplasia induced by barrier disruption. J Invest Dermatol. 2003. 121:142–148.54. Ashida Y, Denda M, Hirao T. Histamine H1 and H2 receptor antagonists accelerate skin barrier repair and prevent epidermal hyperplasia induced by barrier disruption in a dry environment. J Invest Dermatol. 2001. 116:261–265.55. Jeong SK, Ko JY, Seo JT, Ahn SK, Lee CW, Lee SH. Stimulation of epidermal calcium gradient loss and increase in TNF-α and IL-1α expressions by glycolic acid in murine epidermis. Exp Dermatol. 2005. 4:571–579.56. Kim TH, Choi EH, Kang YC, Lee SH, Ahn SK. The effects of topical α-hydroxyacids on the normal skin barrier of hairless mice. Br J Dermatol. 2001. 144:267–273.57. Jeong SK, Kim S, Lee EH, Choi EH, Ahn SK, Lee SH. Comparison of the effect of various chemical peeling agents on the skin barrier. Korean J Dermatol. 2002. 40:1181–1187.58. Parra JL, Paye M. EEMCO Group. EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol Appl Skin Physiol. 2003. 16:188–202.59. Fluhr JW, Behne MJ, Brown BE, Moskowitz DG, Seldel C, Man MQ, et al. Stratum corneym acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiproter-1 acidify neonatal rat stratum corneum. J Invest Dermatol. 2004. 122:320–329.60. Bibel DJ, Miller SJ, Brown BE, Pandey BB, Elias PM, Shinefield HR, et al. Antimicrobial activity of stratum corneum lipids from normal and essential fatty acid-deficiency mice. J Invest Dermatol. 1989. 92:632–638.61. Thueson DO, Chan EK, Oechsli LM, Hahn GS. The roles of pH and concentration in lactic acid-induced stimulation of epidermal turnover. Dermatol Surg. 1998. 24:641–645.62. Rippke F, Schreiner V, Doering T, Maibach HI. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus aureus. Am J Clin Dermatol. 2004. 5:217–223.63. Korting HC, Hubner K, Greiner K, Hamm G, Barun-Falco O. Differences in the skin surface pH and bacterial microflora due to long-term application of synthetic detergent preparations of pH 5.5 and pH 7.0. Results of a crossover trial in healthy volunteers. Acta Derm Venereol. 1990. 70:429–431.64. Berg RW, Milligan MC, Sarbaugh FC. Association of skin wetness and pH with diaper dermatitis. Pediatr Dermatol. 1994. 11:18–20.65. Ferrazzini G, Kaiser RR, Hirsig Cheng SK, Wehrli M, Della Casa V, Pohlig G, et al. Microbiological aspects of diaper dermatitis. Dermatology. 2003. 206:136–141.66. Mauro TM. Eilas PM, Feingold KR, editors. SC pH: measurement, origins, and functions. Skin Barrier. 2006. New York: Taylor & Francis Group;223–230.67. Hachem JP, Behne M, Aronchik I, Demerjian M, Feingold KR, Elias PM, et al. Extracellular pH controls NHE1 expression in epidermis and keratinocytes: implications for barrier repair. J Invest Dermatol. 2005. 125:790–797.68. Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001. 117:44–51.69. Fluhr JW, Crumrine D, Man MQ, Moskowitz DG, Elias PM, Feingold KR. Topical liver X receptor activators accelerate postnatal acidification of stratum corneum and improve function in the neonate. J Invest Dermatol. 2005. 125:1206–1214.70. Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, et al. Degradation of corneodesmosome proteins by two serine protease of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004. 122:1235–1244.71. Brattsand M, Stefansson K, Lunhd C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005. 124:198–203.72. Barel AO, Lambrecht R, Clarys P, Morrison BM, Paye M. A comparative study of the effects on the skin of a classical bar soap and a syndet cleansing bar in normal use conditions and in the soap chamber test. Skin Res Technol. 2001. 7:98–104.73. Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Phamacol Rev. 2001. 53:245–282.74. Hachem J, Uchida Y, Crumrine D, Choi E, Houben E, Brown BE, et al. Serine protease-induced alterations in permeability barrier homeostasis are mediated by protease-activated receptor 2. J Invest Dermatol. 2005. 124:A58.75. Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. J Neurosci. 2003. 23:6176–6180.76. Hong JH, Lee SI, Kim KE, Yong TA, Seo JT, Sohn MH, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol. 2004. 113:315–319.77. Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002. 169:4572–4578.78. Jeong SK, Jeon JE, Kim HJ, Ahn SK, Choi EH, Cho IS, et al. Aeroallergens, as a protease activated receptor-2 activator, delayed epidermal permeability barrier recovery. (under submission).79. Bouwstra JA, Honeywell-Nguyen L, Gooris GS, Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Progress Lipid Res. 2003. 42:1–36.80. Kashiwaga M, Ohba M, Chida K, Kuroki T. Protein kinase C eta (PKC eta): its involvement in keratinocyte differentiation. J Biochem (Tokyo). 2002. 132:853–857.81. Chavanas S, Bodemar T, Rochat A, Hamel-Teillac D, Ali M, Irvine AD, et al. Mutations in SPINK5, encoding a serine protease inhibitorm cause Netherton syndrome. Nat Genet. 2000. 25:141–142.82. Imokawa G, Abe A, Jin K, Higari Y, Kawashima M, Hidano A. Decreased levels of ceremides in stratum corneum of atopic dermatitis: an etiological factor in atopic dermatitis. J Invest Dermatol. 1991. 96:523–526.83. Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991. 283:219–223.84. Bouwstra JA, Gooris GS, Dubbelaar FER, Weerheim AM, Ijzerman AP, Ponec M. Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J Lipids Res. 1998. 39:186–196.85. Pilgram GSK, Vissers DCJ, van der Meulen H, Pavel S, Lavijsen SPM, Bouwstra JA, et al. Aberrant lipid organization in stratum corneum of parients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001. 117:710–717.86. Hara J, Higuchi K, Okamoto R, Kawashima M, Imokawa G. High-expression of sphigomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2002. 115:406–413.87. Ishibashi M, Arikawa J, Okamoto R, Kawashima M, Takagi Y, Ohguchi K, et al. Abnormal expression of the novel epidermal enzyme, glucosylceramide deacylase, and the accumulation of its enzymatic reaction product, glucosylsphingosine, in the skin of patients with atopic dermatitis. Lab Invest. 2003. 83:397–408.88. Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphigosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002. 119:433–439.89. Sugiura H, Ebise H, Tazawa T, Tanaka K, Sugiura Y, Uehara M, et al. Large-scale DNA microarray analysis of atopic skin lesions shows overexpression of an epidermal differentiation gene cluster in the alternative pathway and lack of protective gene expression in the cornified envelope. Br J Dermatol. 2005. 152:146–149.90. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006. 38:441–446.91. Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006. 38:337–342.92. Ginger RS, Blachford S, Rowland J, Rowson M, Harding CR. Filaggrin repeat number polymorphism is associated with a dry skin phenotype. Arch Dermatol Res. 2005. 297:235–241.93. Hudson T. Skin barrier function and allergic risk. Nat Genet. 2006. 38:399–400.94. Tagami H, Yoshikuni K. Interrelationship between water barrier and reservoir function in pathologic stratum corneum. Arch Dermatol. 1985. 121:642–645.95. Motta S, Monti M, Sesana S, Mellesi L, Ghidoni R, Caputo R. Abnormality in water barrier function in psoriasis: role of ceramide fraction. Arch Dermatol Res. 1994. 130:452–456.96. Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. J Invest Dermatol. 1996. 107:558–564.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atopic Dermatitis and Epidermal Barrier
- Atopic dermatitis and skin barrier dysfunction
- The Influence of Physiologic Lipid Containing Moisturizer on the Normal Skin Barrier
- Role of Micronutrients in Skin Health and Function
- Skin Barrier Dysfunction in the Scalp, Nails, and Lips in Patients with Atopic Dermatitis