J Korean Med Sci.
2010 Jan;25(1):110-116. 10.3346/jkms.2010.25.1.110.
GnRH Agonist Therapy to Protect Ovarian Function in Young Korean Breast Cancer Patients
- Affiliations
-
- 1Department of Obstetrics & Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dooseok.choi@samsung.com
- 2Department of Hematology & Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1713840
- DOI: http://doi.org/10.3346/jkms.2010.25.1.110
Abstract
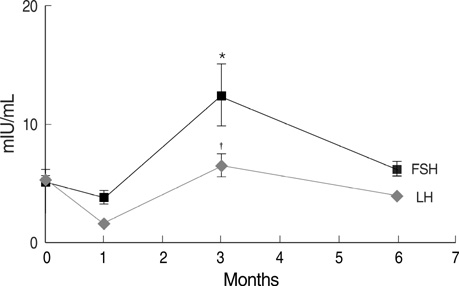
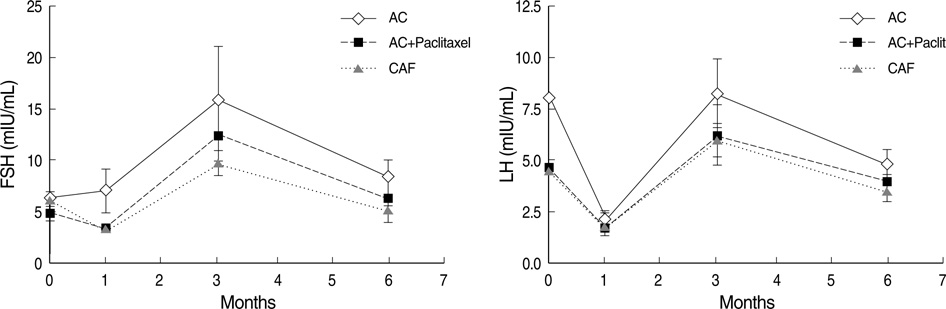
- The increased survival of patients with breast cancer has given rise to other problems associated with the complications of chemotherapy. One major complication is premature ovarian failure, an especially harmful outcome for women of reproductive age. This study was performed to evaluate the efficacy of GnRH agonist (GnRHa) treatment on protecting ovarian function in young breast cancer patients (30.59+/-5.1 yr) receiving chemotherapy after surgery. Twenty-two women were enrolled and given subcutaneous injections of leuprolide acetate (3.75 mg) every 4 weeks during chemotherapy. Follow-up laboratory tests (luteinizing hormone [LH], follicle stimulating hormone [FSH], and estradiol) were performed 1, 3, and 6 months after chemotherapy. Menstruation patterns and clinical symptoms were followed up for a mean duration of 35.6+/-1.7 months. FSH and LH levels were normal in all patients 6 months after completing chemotherapy (8.0+/-5.3, 4.4+/-2.7 mIU/mL, respectively). During follow-up, none of the patients complained of menopausal symptoms and 81.8% experienced recovery of menstruation. This report is the first trial of GnRHa as a treatment modality to protect ovarian function during adjuvant chemotherapy in young Korean breast cancer patients.
Keyword
MeSH Terms
-
Adult
Antineoplastic Agents/adverse effects/therapeutic use
Antineoplastic Agents, Hormonal/therapeutic use
Breast Neoplasms/diagnosis/*drug therapy/surgery
Combined Modality Therapy
Cyclophosphamide/adverse effects/therapeutic use
Doxorubicin/adverse effects/therapeutic use
Female
Follicle Stimulating Hormone/analysis
Gonadotropin-Releasing Hormone/*agonists
Humans
Leuprolide/administration & dosage
Luteinizing Hormone/analysis
Menstruation
Ovarian Function Tests
Primary Ovarian Insufficiency/etiology/*prevention & control
Republic of Korea
Tamoxifen/therapeutic use
Time Factors
Antineoplastic Agents
Antineoplastic Agents, Hormonal
Tamoxifen
Doxorubicin
Gonadotropin-Releasing Hormone
Cyclophosphamide
Leuprolide
Luteinizing Hormone
Follicle Stimulating Hormone
Figure
Reference
-
1. Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol. 2003. 4:251–254.
Article2. Ataya K, Moghissi K. Chemotherapy-induced premature ovarian failure: mechanisms and prevention. Steroids. 1989. 54:607–626.
Article3. Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996. 11:1620–1626.4. Pereyra Pacheco B, Mendez Ribas JM, Milone G, Fernandez I, Kvicala R, Mila T, Di Noto A, Contreras Ortiz O, Pavlovsky S. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: a preliminary report. Gynecol Oncol. 2001. 81:391–397.5. Recchia F, Saggio G, Amiconi G, Di Blasio A, Cesta A, Candeloro G, Rea S. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006. 106:514–523.
Article6. Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embryo, oocytes or ovaries. Oncologist. 2007. 12:1044–1054.7. Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999. 17:2365–2370.
Article8. Chapman RM, Sutcliffe SB, Malpas JS. Cytotoxic-induced ovarian failure in women with Hodgkins disease. I. Hormone function. JAMA. 1979. 242:1877–1881.
Article9. Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996. 14:1718–1729.
Article10. Santoro A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, Pagnoni AM, Bonfante V, Musumeci R, Crippa F. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin's disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987. 5:27–37.
Article11. Bokemeyer C, Schmoll HJ, van Rhee J, Kuczyk M, Schuppert F, Poliwoda H. Long-term gonadal toxicity after therapy for Hodgkin's and non-Hodgkin's lymphoma. Ann Hematol. 1994. 68:105–110.
Article12. Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormonereleasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995. 52:365–372.13. Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J Soc Gynecol Investig. 1999. 6:229–239.
Article14. Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1997. 39:1403–1409.
Article15. Nabholtz J, Peinkowski T, Mackey J. Phase III trial comparing TAC with RAC in the adjuvant treatment of node positive breast cancer patients:interim analysis of the BCIRG001 study. Pro Am Soc Clin Oncol. 2002. 21:36a.16. Kil WJ, Ahn SD, Shin SS, Lee WS, Choi EK, Kim JH, Son BH, Ahn SH, Kim WK, Kim SB. Treatment-induced menstrual changes in very young (<35 years old) breast cancer patients. Breast Cancer Res Treat. 2006. 96:245–250.17. Arriagada R, Le MG, Spielmann M, Mauriac L, Bonneterre J, Namer M, Delozier T, Hill C, Tursz T. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol. 2005. 16:389–396.
Article18. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006. 24:2917–2931.19. Blumenfeld Z. Ovarian rescue/protection from chemotherapeutic agents. J Soc Gynecol Investig. 2001. 8:S60–S64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum cholesterol changes by duration of GnRH-agonist therapy in premenopausal women with breast cancer
- Quality of life according to add-back therapy during GnRH agonist treatment in endometriosis patients
- The feasibility and efficacy of gonadotropin-releasing hormone agonists for prevention of chemotherapy induced ovarian failure in patient with gynecological malignancies
- Serum Anti-Müllerian Hormone Levels in Precocious Puberty Girls according to Stage of GnRH Agonist Treatment
- Fertility Rates in Young Korean Breast Cancer Patients Treated with Gonadotropin-Releasing Hormone and Chemotherapy