J Korean Med Sci.
2007 Oct;22(5):784-790. 10.3346/jkms.2007.22.5.784.
Evaluation of the Broth Microdilution Method Using 2,3-Diphenyl-5-thienyl-(2)-tetrazolium Chloride for Rapidly Growing Mycobacteria Susceptibility Testing
- Affiliations
-
- 1Department of Laboratory Medicine, School of Medicine, Pusan National University, Busan, Korea. CCHL@pusan.ac.kr
- 2Department of Internal Medicine, School of Medicine, Pusan National University, Busan, Korea.
- 3Department of Laboratory Medicine, Donga University College of Medicine, Busan, Korea.
- 4Department of Laboratory Medicine, Ulsan University Hospital, Ulsan, Korea.
- 5Korean Institute of Tuberculosis, Seoul, Korea.
- 6Medical Research Institute, Pusan National University, Busan, Korea.
- 7MRC for Ischemic Tissue Regeneration, Pusan National University, Busan, Korea.
- KMID: 1713281
- DOI: http://doi.org/10.3346/jkms.2007.22.5.784
Abstract
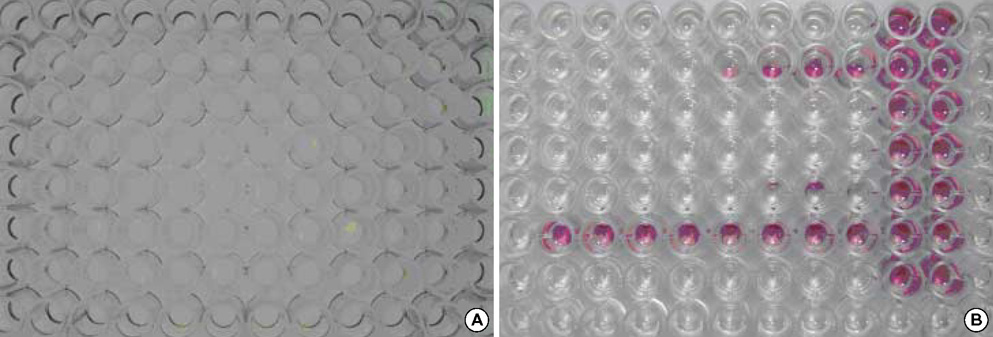
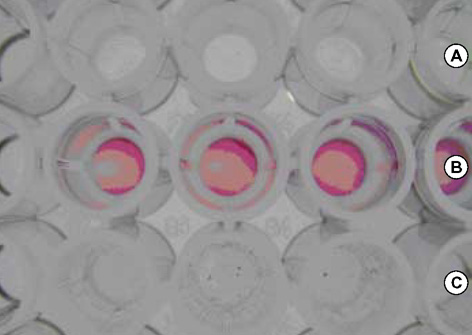
- As the incidence of nontuberculous mycobacterial infection has been increasing recently in Korea, the importance of drug susceptibility test for clinical isolates of mycobacteria has become larger. In this study we determined the antimicrobial susceptibility patterns of clinical isolates of M. fortuitum and M. abscessus in Korea, and evaluated the efficacy of a modified broth microdilution method using 2,3-diphenyl-5-thienyl-(2)-tetrazolium chloride (STC), in terms of its ability to provide accurate and easy-to-read minimal inhibitory concentration (MIC) endpoints for the susceptibility testing of rapidly growing mycobacteria. Most isolates of M. fortuitum and M. abscessus in Korea are susceptible or intermediately susceptible to amikacin, cefoxitin, ciprofloxacin, and clarithromycin. Many isolates of M. fortuitum are susceptible to doxycycline, sulfamethoxazole, and imipenem, while many M. abscessus isolates are resistant to these drugs. In the present study, the modified broth microdilution method using STC was found to be reliable, easy to read, and inexpensive for M. fortuitum and M. abscessus susceptibility testing. The modified colorimetric MIC testing method using STC was proven to be a useful surrogate for RGM antibiotic susceptibility testing.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/pharmacology
Cefoxitin/pharmacology
Chemistry, Pharmaceutical/methods
Ciprofloxacin/pharmacology
Clarithromycin/pharmacology
Colorimetry/methods
Drug Resistance, Bacterial
Korea
*Microbial Sensitivity Tests
Mycobacterium/*metabolism
Mycobacterium fortuitum/*metabolism
Tetrazolium Salts/*pharmacology
Figure
Cited by 2 articles
-
Clarithromycin Susceptibility Testing of Mycobacterium avium Complex Using 2,3-Diphenyl-5-thienyl-(2)-tetrazolium Chloride Microplate Assay with Middlebrook 7H9 Broth
Young Kil Park, Won-Jung Koh, Shin Ok Kim, Sonya Shin, Bum Joon Kim, Sang-Nae Cho, Sun Min Lee, Chulhun L. Chang
J Korean Med Sci. 2009;24(3):511-512. doi: 10.3346/jkms.2009.24.3.511.Detection of Rifampicin Resistance in Mycobacterium tuberculosis by Using Middlebrook 7H9 Broth Medium with 2,3-Diphenyl-5-Thienyl-(2)-Tetrazolium Chloride
Sun Min Lee, Kyung Jun Kim, Chulhun L. Chang
Ann Clin Microbiol. 2018;21(3):47-50. doi: 10.5145/ACM.2018.21.3.47.
Reference
-
1. Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004. 32:257–270.
Article2. Choudhri S, Manfreda J, Wolfe J, Parker S, Long R. Clinical significance of nontuberculous mycobacteria isolates in a Canadian tertiary care center. Clin Infect Dis. 1995. 21:128–133.
Article3. Hosker HS, Lam CW, Ng TK, Ma HK, Chan SL. The prevalence and clinical significance of pulmonary infection due to non-tuberculous mycobacteria in Hong Kong. Respir Med. 1995. 89:3–8.
Article4. Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002. 23:553–567.5. Scientific Committee in Korean Academy of Tuberculosis and Respiratory diseases. National survey of mycobacterial diseases other than tuberculosis in Korea. Tuberc Respir Dis. 1995. 42:277–294.6. Lee JY, Choi HJ, Lee HY, Joung EY, Huh JW, Oh YM, Lee SD, Kim WS, Kim DS, Kim WD, Shim TS. Recovery rate and characteristics of nontuberculous mycobacterial isolates in a university hospital in Korea. Tuberc Respir Dis. 2005. 58:385–391.
Article7. Koh WJ, Kwon OJ, Lee KS. Nontuberculous mycobacterial pulmonary diseases in immunocompetent patients. Korean J Radiol. 2002. 3:145–157.
Article8. Lew WJ. Clinical manifestations and epidermiology of nontuberculous mycobacterial infection in Korea. Tuberc Respir Dis. 2002. 53:83–87.9. Griffith DE. Mycobacteria as pathogens of respiratory infection. Infect Dis Clin North Am. 1998. 12:593–611.
Article10. Lee HW, Kim MN, Shim TS, Bia GH, Pai CH. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients. Tuberc Respir Dis. 2002. 53:173–182.
Article11. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, Bai GH. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006. 129:341–348.
Article12. Yim JJ, Han SK. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases. J Korean Med Assoc. 2005. 48:563–570.
Article13. Falkinham JO 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996. 9:177–215.
Article14. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997. 156:S1–S25.15. Clinical and Laboratory Standards Institute. CLSI document M24-A. Susceptibility testing of mycobacteria, Norcardiae, and Other Aerobic Actinomycetes; Approved Standard. 2003. Wayne, Pa. USA: CLSI.16. Clinical and Laboratory Standards Institute. CLSI document M100-S15. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement. 2005. Wayne, Pa. USA: CLSI.17. Clinical and Laboratory Standards Institute. CLSI document M7-A6. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard-sixth edition. 2003. Wayne, Pa. USA: CLSI.18. Yamane N, Oiwa T, Kiyota T, Saitoh H, Sonoda T, Tosaka M, Nakashima M, Fukunaga H, Masaki T, Miyagawa K, Miyagoe M, Okazawa Y. Multicenter evaluation of a colorimetric microplate antimycobacterial susceptibility test: comparative study with the NCCLS M24-P. Rinsho Byori. 1996. 44:456–464.19. Lee S, Kong DH, Yun SH, Lee KR, Lee KP, Franzblau SG, Lee EY, Chang CL. Evaluation of a modified antimycobacterial susceptibility test using Middlebrook 7H10 agar containing 2,3-diphenyl-5-thienyl-(2)-tetrazolium chloride. J Microbiol Methods. 2006. 66:548–551.
Article20. Shin JH, Choi JC, Lee JN, Kim HH, Lee EY, Chang CL. Evaluation of a colorimetric antifungal susceptibility test by using 2,3-diphenyl-5-thienyl-(2)-tetrazolium chloride. Antimicrob Agents Chemother. 2004. 48:4457–4459.
Article21. Lee H, Park HJ, Cho SN, Bai GH, Kim SJ. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol. 2000. 38:2966–2971.22. Butler WR, Jost KC Jr, Kilburn JO. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991. 29:2468–2472.
Article23. Butler WR, Floyd MM, Silcox V, Cage G, Desmond E, Duffey PS, Guthertz LS, Gross WM, Kenneth C, Jost J, Ramos LS, Thibert L, Warren N. Mycolic acid pattern standards for HPLC identification of mycobacteria. 1999. Atlanta: Centers for Deasese Control and Prevention.24. Clinical and Laboratory Standards Institute. Development of in vitro susceptibility testing criteria and quality controls parameters, Approved guideline M23-A2. 2001. Wayne: CLSI.25. Brown BA, Wallace RJ Jr, Onyi GO, De Rosas V, Wallace RJ 3rd. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonaelike organisms. Antimicrob Agents Chemother. 1992. 36:180–184.
Article26. Wallace RJ Jr, Bedsole G, Sumter G, Sanders CV, Steele LC, Brown BA, Smith J, Graham DR. Activities of ciprofloxacin and ofloxacin against rapidly growing mycobacteria with demonstration of acquired resistance following single-drug therapy. Antimicrob Agents Chemother. 1990. 34:65–70.
Article27. Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000. 31:1209–1215.28. Woods GL, Bergmann JS, Witebsky FG, Fahle GA, Wanger A, Boulet B, Plaunt M, Brown BA, Wallace RJ Jr. Multisite reproducibility of results obtained by the broth microdilution method for susceptibility testing of Mycobacterium abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum. J Clin Microbiol. 1999. 37:1676–1682.29. Rahman M, Kuhn I, Rahman M, Olsson-Liljequist B, Mollby R. Evaluation of a scanner-assisted colorimetric MIC method for susceptibility testing of gram-negative fermentative bacteria. Appl Environ Microbiol. 2004. 70:2398–2403.
Article30. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.
Article31. Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991. 142:257–265.
Article32. Garn H, Krause H, Enzmann V, Drossler K. An improved MTT assay using the electron-coupling agent menadione. J Immunol Methods. 1994. 168:253–256.
Article33. Hawser SP, Norris H, Jessup CJ, Ghannoum MA. Comparison of a 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-t etrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J Clin Microbiol. 1998. 36:1450–1452.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clarithromycin Susceptibility Testing of Mycobacterium avium Complex Using 2,3-Diphenyl-5-thienyl-(2)-tetrazolium Chloride Microplate Assay with Middlebrook 7H9 Broth
- Detection of Rifampicin Resistance in Mycobacterium tuberculosis by Using Middlebrook 7H9 Broth Medium with 2,3-Diphenyl-5-Thienyl-(2)-Tetrazolium Chloride
- Antifungal Susceptibility Testing of Dermatophytes
- Evaluation of a Colorimetric Broth Microdilution Method for Antimicrobial Susceptibility Testing Using 2,3,5-Triphenyltetrazolium Chloride
- Comparison of Modified Broth Microdilution Method, E test, and Disk Diffusion Method for Antimicrobial Susceptibility Testing of Helicobacter pylori