J Bacteriol Virol.
2007 Sep;37(3):137-146. 10.4167/jbv.2007.37.3.137.
Elevated Levels of Interferon-inducible Protein-10 (IP)-10/CXCL10, but not of Interferon-gamma, in Patients with Pulmonary Tuberculosis
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chungnam National University, Daejeon 301-747, Republic of Korea. hayoungj@cnu.ac.kr
- 2Department of Internal Medicine, College of Medicine, Konyang University, Daejeon 302-718, Republic of Korea.
- 3Department of Microbiology, College of Medicine, Konyang University, Daejeon 302-718, Republic of Korea.
- 4Infection Signaling Network Research Center, College of Medicine, Chungnam National University, Daejeon 301-747, Republic of Korea.
- KMID: 1513568
- DOI: http://doi.org/10.4167/jbv.2007.37.3.137
Abstract
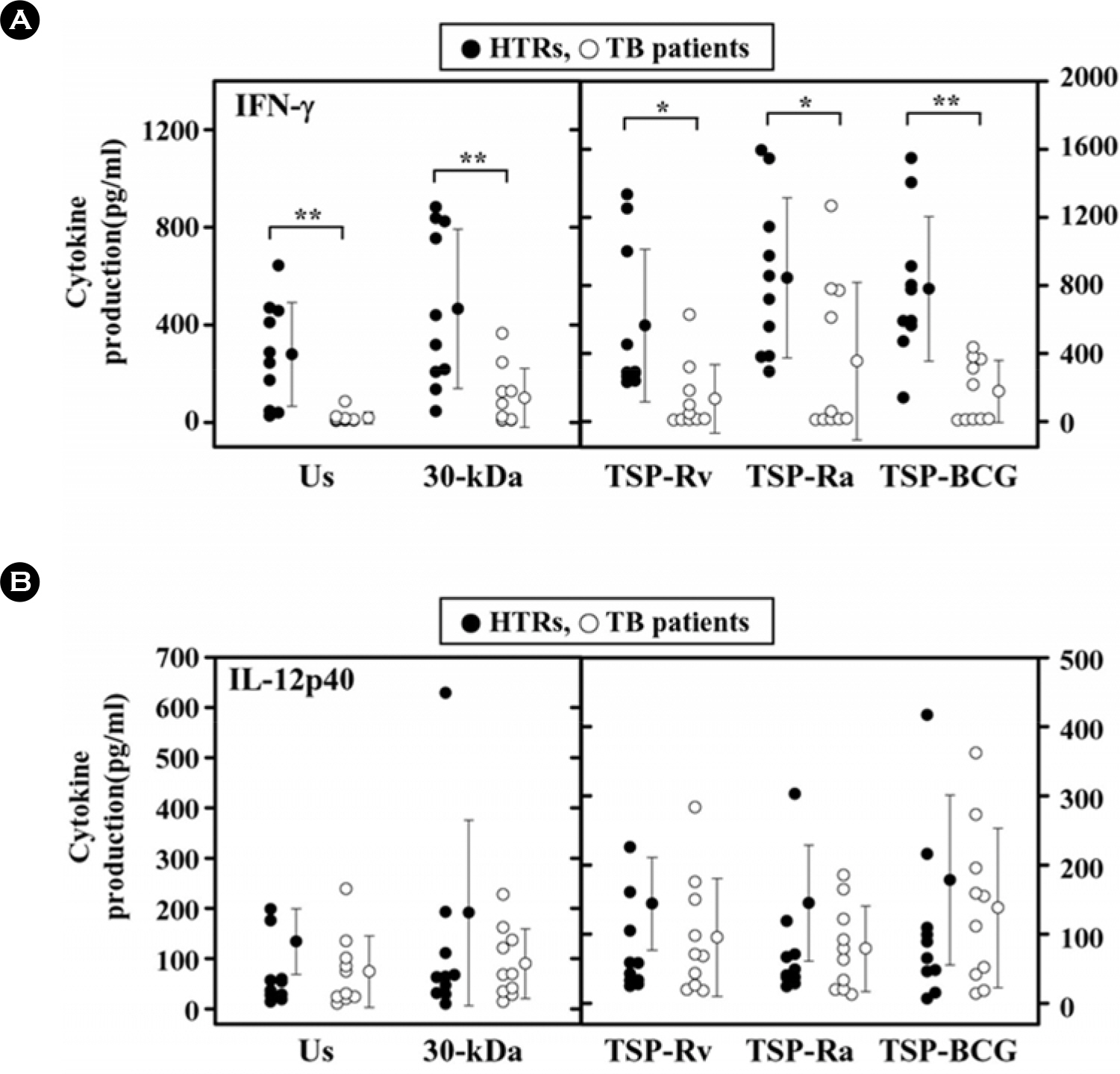
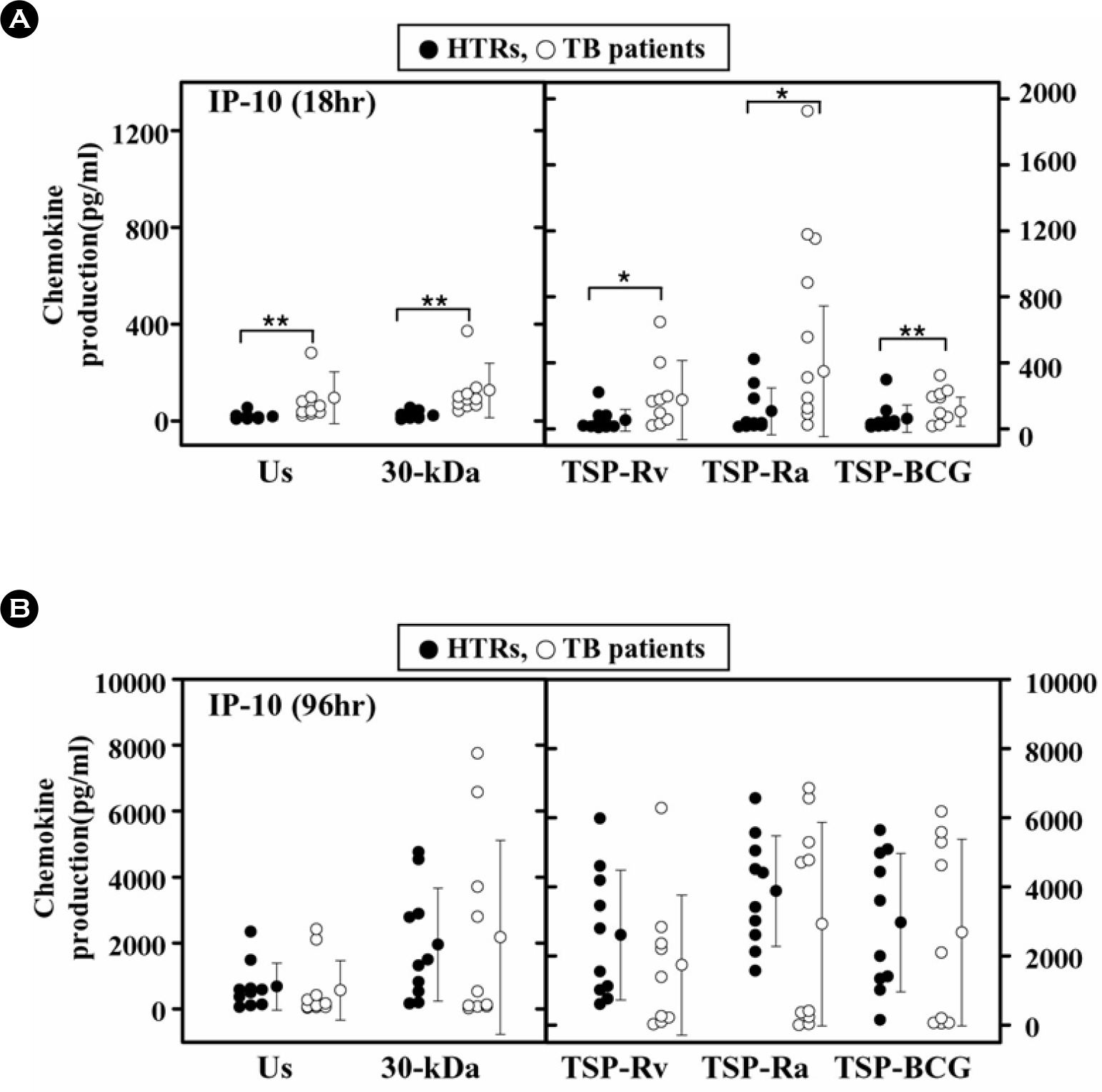
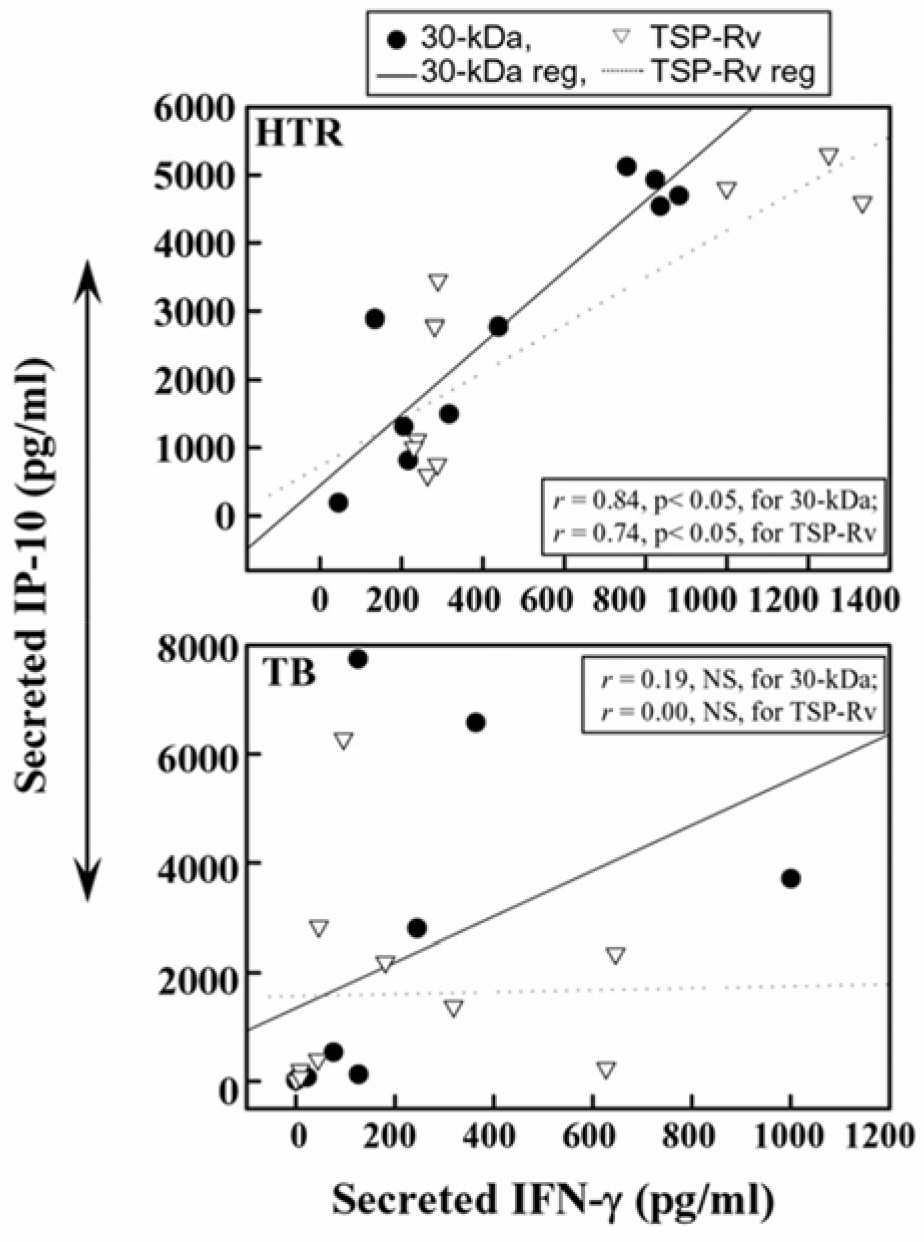
- Mycobacterial strains are potent inducers of cytokines/chemokines by mononuclear phagocytes, which constitute an important cellular component of the first line of defense in the innate immune system. Interferon (IFN)-gamma-inducible protein (IP-10 or CXCL10) is a potent chemoattractant; however, little is known about the IP-10 profiles attributable to the Th1 regulation associated with active tuberculosis (TB). In this study, we investigated the production of IP-10, interleukin (IL)-12 p40, and IFN-gamma by the peripheral blood mononuclear cells (PBMCs) of patients with active pulmonary TB in response to in vitro stimulation with Triton X-100 soluble proteins (TSPs) or the 30-kDa antigen. The TSP antigens used in the present study were isolated and purified from Mycobacterium tuberculosis H37Rv (virulent strain), M. tuberculosis H37Ra (avirulent strain), and Mycobacterium bovis BCG. The results were compared with those obtained for healthy tuberculin reactors (HTRs). Concordant with earlier studies, IFN-gamma production was significantly depressed in the PBMCs from TB patients compared with those in the HTR group. However, the IP-10 levels in the PBMCs from TB patients were significantly elevated 18 h after stimulation compared to those in the PBMCs from HTRs. IP-10 release was correlated in a significant manner with the release of IFN-gamma in the HTRs, but this was not the case for the TB patients. Collectively, these data suggest that TB patients show altered regulation of Th1-driving cytokine and chemokine production in response to a variety of mycobacterial antigens.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Mycobacterium bovis Bacillus Calmette-Guerin (BCG) and BCG-based Vaccines Against Tuberculosis
Seung Bin Cha, Sung Jae Shin
J Bacteriol Virol. 2014;44(3):236-243. doi: 10.4167/jbv.2014.44.3.236.
Reference
-
References
1). Aung H, Toossi Z, Wisnieski JJ, Wallis RS, Culp LA, Phillips NB, Phillips M, Averill LE, Daniel TM, Ellner JJ. Induction of monocyte expression of tumor necrosis factor alpha by the 30-kD alpha antigen of Mycobacterium tuberculosis and synergism with fibronectin. J Clin Invest. 98:1261–1268. 1996.2). Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, Benagiano M, D'Elios MM, Mantovani A, Del Prete G. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 7:1–8. 2005.3). Berthier-Vergnes O, Bermond F, Flacher V, Massacrier C, Schmitt D, Péguet-Navarro J. TNF-alpha enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 579:3660–3668. 2005.4). Boom WH, Canaday DH, Fulton SA, Gehring AJ, Rojas RE, Torres M. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis (Edinb). 83:98–106. 2003.5). Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 20:F1–F7. 2006.
Article6). Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 178:2243–2247. 1993.
Article7). Dlugovitzky D, Bay ML, Rateni L, Urízar L, Rondelli CF, Largacha C, Farroni MA, Molteni O, Bottasso OA. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol. 49:210–217. 1999.8). Ferrero E, Biswas P, Vettoretto K, Ferrarini M, Uguccioni M, Piali L, Leone BE, Moser B, Rugarli C, Pardi R. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leukocyte subpopulations: focus on gammadelta cells. Immunology. 108:365–374. 2003.9). Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 19:93–129. 2001.
Article10). Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 178:2249–2254. 1993.11). Goodridge HS, Harnett W, Liew FY, Harnett MM. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology. 109:415–425. 2003.
Article12). Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, Peters P, Ellner JJ. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 180:2069–2073. 1999.13). Jo EK, Kim HJ, Lim JH, Min D, Song Y, Song CH, Paik TH, Suhr JW, Park JK. Dysregulated production of interferon-gamma, interleukin-4 and interleukin-6 in early tuberculosis patients in response to antigen 85B of Mycobacterium tuberculosis. Scand J Immunol. 51:209–217. 2000.14). Juffermans NP, Verbon A, van Deventer SJ, van Deutekom H, Belisle JT, Ellis ME, Speelman P, van der Poll T. Elevated chemokine concentrations in sera of human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients with tuberculosis: a possible role for mycobacterial lipoarabinomannan. Infect Immun. 67:4295–4297. 1999.
Article15). Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 166:1098–108. 1987.
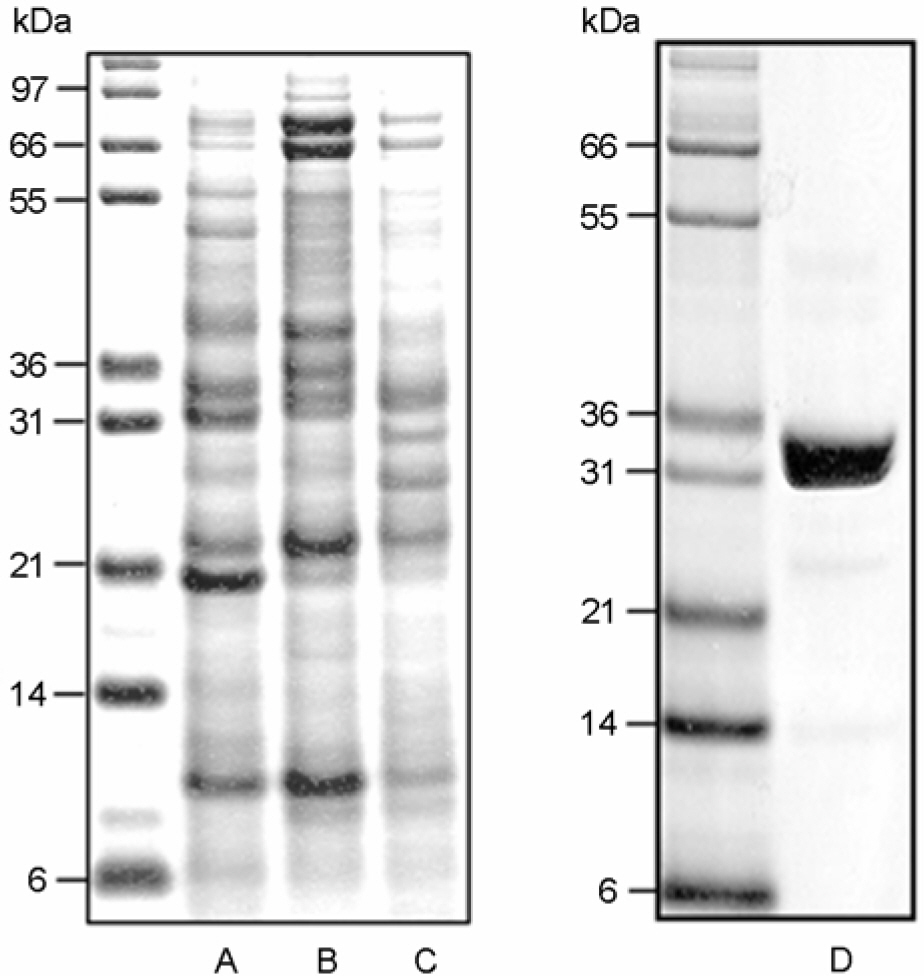
Article16). Kim HJ, Jo EK, Park JK, Lim JH, Min D, Paik TH. Isolation and partial characterisation of the Triton X-100 solubilised protein antigen from Mycobacterium tuberculosis. J Med Microbiol. 48:585–591. 1999.17). Lahrtz F, Piali L, Nadal D, Pfister HW, Spanaus KS, Baggiolini M, Fontana A. Chemotactic activity on mononuclear cells in the cerebrospinal fluid of patients with viral meningitis is mediated by interferon-gamma inducible protein-10 and monocyte chemotactic protein-1. Eur J Immunol. 27:2484–2489. 1997.18). Lee BY, Horwitz MA. T-cell epitope mapping of the three most abundant extracellular proteins of Mycobacterium tuberculosis in outbred guinea pigs. Infect Immun. 67:2665–2670. 1999.19). Lee JS, Son JW, Jung SB, Kwon YM, Yang CS, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Ex vivo responses for interferon-gamma and proinflammatory cytokine secretion to low-molecular-weight antigen MTB12 of Mycobacterium tuberculosis during human tuberculosis. Scand J Immunol. 64:145–154. 2006.20). Lee JS, Song CH, Kim CH, Kong SJ, Shon MH, Suhr JW, Jung SS, Lim JH, Kim HJ, Park JK, Paik TH, Jo EK. Depressed interleukin-12 production by peripheral blood mononuclear cells after in vitro stimulation with the 30-kDa antigen in recurrent pulmonary tuberculosis patients. Med Microbiol Immunol. 192:61–69. 2003a.
Article21). Lee JS, Song CH, Lim JH, Kim HJ, Park JK, Paik TH, Kim CH, Kong SJ, Shon MH, Jung SS, Jo EK. The production of tumour necrosis factor-alpha is decreased in peripheral blood mononuclear cells from multidrug-resistant tuberculosis patients following stimulation with the 30-kDa antigen of Mycobacterium tuberculosis. Clin Exp Immunol. 132:443–449. 2003b.22). Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J Exp Med. 198:1265–1276. 2003.23). Lokensgard JR, Hu S, Sheng W, vanOijen M, Cox D, Cheeran MC, Peterson PK. Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J Neurovirol. 7:208–219. 2001.24). Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J Exp Med. 166:1084–1097. 1987.
Article25). Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 315:672–676. 1985.26). Ma X, Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol. 79:55–92. 2001.27). Okamoto M, Kawabe T, Iwasaki Y, Hara T, Hashimoto N, Imaizumi K, Hasegawa Y, Shimokata K. Evaluation of interferon-gamma, interferon-gamma-inducing cytokines, and interferon-gamma-inducible chemokines in tuberculous pleural effusions. J Lab Clin Med. 145:88–93. 2005.28). Prabhakar S, Qiao Y, Canova A, Tse DB, Pine R. IFN-αβ secreted during infection is necessary but not sufficient for negative feedback regulation of IFN-αβ signaling by Mycobacterium tuberculosis. J Immunol. 174:1003–1012. 2005.29). Raja A. Immunology of tuberculosis. Indian J Med Res. 120:213–232. 2004.30). Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H, Nelwan RH, Marzuki S, van der Meer JW, van Crevel R, van de Vosse E, Ottenhoff TH. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun. 75:820–829. 2007.
Article31). Song CH, Kim HJ, Park JK, Lim JH, Kim UO, Kim JS, Paik TH, Kim KJ, Suhr JW, Jo EK. Depressed interleukin-12 (IL-12), but not IL-18, production in response to a 30- or 32-kilodalton mycobacterial antigen in patients with active pulmonary tuberculosis. Infect Immun. 68:4477–4484. 2000.
Article32). Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 163:1162–1172. 1986.
Article33). Torres M, Herrera T, Villareal H, Rich EA, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 66:176–180. 1998.34). Yang CS, Lee JS, Jung SB, Oh JH, Song CH, Kim HJ, Park JK, Paik TH, Jo EK. Differential regulation of interleukin-12 and tumour necrosis factor-alpha by phosphatidylinositol 3-kinase and ERK 1/2 pathways during Mycobacterium tuberculosis infection. Clin Exp Immunol. 143:150–160. 2006.34). WHO. WHO annual report on global TB control-summary. Wkly Epidemiol Rec. 78:122–128. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical characteristics of interferon-gamma-inducible protein of 10 kDa in children with wheezing
- Upregulation of IP-10(CXCL10) mRNA Expression by Interleukin-18
- Association of IL28B Genotypes and Baseline Serum Interferon-γ-Inducible-Protein-10 Levels with Treatment Response in Hepatitis C Virus Patients in China
- Benzylideneacetophenone derivatives attenuate IFN-gamma-induced IP-10/CXCL10 production in orbital fibroblasts of patients with thyroid-associated ophthalmopathy through STAT-1 inhibition
- The clinical significance of interferon inducible protein-10 in patients having chronic hepatitis C with genotype I