Yonsei Med J.
2013 Mar;54(2):494-499. 10.3349/ymj.2013.54.2.494.
Expression and Localization of COMMD1 Proteins in Human Placentas from Women with Preeclampsia
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Konkuk University School of Medicine, Seoul, Korea. 20050024@kuh.ac.kr
- 2Department of Anatomy, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 1503916
- DOI: http://doi.org/10.3349/ymj.2013.54.2.494
Abstract
- PURPOSE
Recently, COMMD1 has been identified as a novel interactor and regulator of hypoxia-inducible factor-1 and nuclear factor kappa B transcriptional activity. The goal of this study was to determine the difference of COMMD1 expression in the placentas of women with normal and preeclamptic (PE) pregnancies.
MATERIALS AND METHODS
Immnoperoxidase and immunofluorescent staining for COMMD1 was performed on nine normal and nine severe PE placental tissues, and COMMD1 mRNA expression was quantified by quantitative reverse transcription polymerase chain reaction.
RESULTS
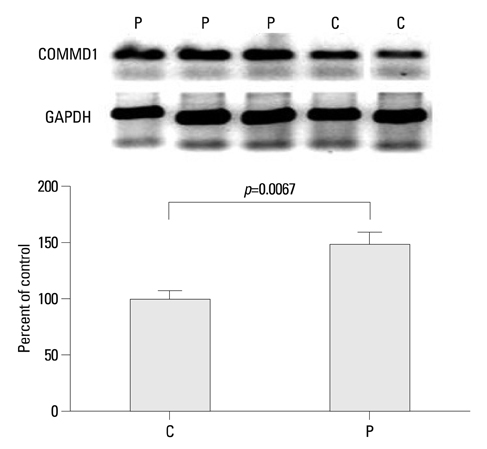
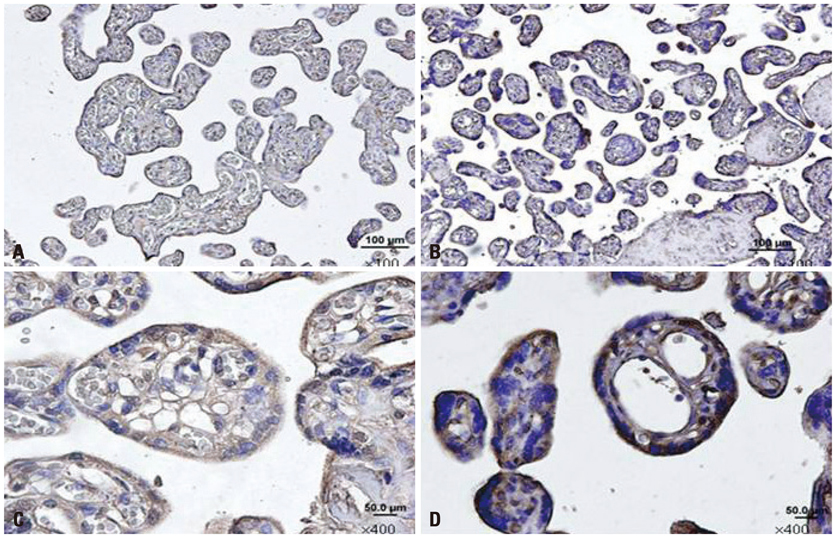
The expression of mRNA of COMMD1 was significantly higher in the study group than in the control group. The immunoreactivity was higher especially in the syncytiotrophoblast of PE placentas than in the control group.
CONCLUSION
This study demonstrated increased placental COMMD1 expression in women with severe preeclampsia compared to that found in women with normal pregnancies, and this finding might contribute to a better understanding of the pathophysiology of preeclampsia.
Keyword
MeSH Terms
Figure
Reference
-
1. Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991. 4:700–708.
Article2. Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998. 179:1359–1375.
Article3. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999. 180(2 Pt 1):499–506.
Article4. Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999. 20:114–118.
Article5. Khong TY, Robertson WB. Coulam CB, Faulk WP, Mclntyre JA, editors. Spiral Artery Disease. Immunological obstetrics. 1992. New York: W.W.Norton & Company;492–501.6. Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997. 37:240–249.
Article7. Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, et al. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem. 2005. 280:22222–22232.
Article8. Ganesh L, Burstein E, Guha-Niyogi A, Louder MK, Mascola JR, Klomp LW, et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003. 426:853–857.
Article9. Maine GN, Mao X, Komarck CM, Burstein E. COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase. EMBO J. 2007. 26:436–447.
Article10. Green DR. Death and NF-kappaB in T cell activation: life at the edge. Mol Cell. 2003. 11:551–552.11. Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002. 3:221–227.12. Perkins ND. The Rel/NF-kappa B family: friend and foe. Trends Biochem Sci. 2000. 25:434–440.13. Silverman N, Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001. 15:2321–2342.14. van de Sluis B, Muller P, Duran K, Chen A, Groot AJ, Klomp LW, et al. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. Mol Cell Biol. 2007. 27:4142–4156.
Article15. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992. 12:5447–5454.
Article16. Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003. 24:199–208.
Article17. Donadio S, Alfaidy N, De Keukeleire B, Micoud J, Feige JJ, Challis JR, et al. Expression and localization of cellular prion and COMMD1 proteins in human placenta throughout pregnancy. Placenta. 2007. 28:907–911.
Article18. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000. 183:S1–S22.19. Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006. 147:1675–1684.
Article20. Tao TY, Liu F, Klomp L, Wijmenga C, Gitlin JD. The copper toxicosis gene product Murr1 directly interacts with the Wilson disease protein. J Biol Chem. 2003. 278:41593–41596.
Article21. Lowndes SA, Harris AL. Copper chelation as an antiangiogenic therapy. Oncol Res. 2004. 14:529–539.
Article22. Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis--clinical implications. J Trace Elem Med Biol. 2004. 18:1–8.
Article23. Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002. 62:4854–4859.24. Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta. 2000. 21:Suppl A. S16–S24.25. Sánchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabéu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. 2002. 277:43799–43808.
Article26. Rajakumar A, Whitelock KA, Weissfeld LA, Daftary AR, Markovic N, Conrad KP. Selective overexpression of the hypoxia-inducible transcription factor, HIF-2alpha, in placentas from women with preeclampsia. Biol Reprod. 2001. 64:499–506.
Article27. Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review. Placenta. 2002. 23:Suppl A. S47–S57.
Article28. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004. 350:672–683.
Article29. Foidart JM, Schaaps JP, Chantraine F, Munaut C, Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia--a step forward but not the definitive answer. J Reprod Immunol. 2009. 82:106–111.
Article30. Abe E, Matsubara K, Oka K, Kusanagi Y, Ito M. Cytokine regulation of intercellular adhesion molecule-1 expression on trophoblasts in preeclampsia. Gynecol Obstet Invest. 2008. 66:27–33.
Article31. Wruck CJ, Huppertz B, Bose P, Brandenburg LO, Pufe T, Kadyrov M. Role of a fetal defence mechanism against oxidative stress in the aetiology of preeclampsia. Histopathology. 2009. 55:102–106.
Article32. Shin JK, Han KA, Kang MY, Kim YS, Park JK, Choi WJ, et al. Expression of clusterin in normal and preeclamptic placentas. J Obstet Gynaecol Res. 2008. 34:473–479.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential expression of Matrix Metalloproteinase (MMP)-2, -9 in normal and severe preeclamptic human placentas
- Expression of Aquaporin-4 in Placenta of Preeclampsia
- Expression of Aquaporin-8 in Placenta of Preeclampsia
- Expression and Localization of the PlGF mRNA in Preeclamptic Placentas
- Placental expression of D6 decoy receptor in preeclampsia