Yonsei Med J.
2013 Mar;54(2):315-320. 10.3349/ymj.2013.54.2.315.
Precommissural Fornix in the Human Brain: A Diffusion Tensor Tractography Study
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Korea. strokerehab@hanmail.net
- 2Department of Physical Therapy, Yeungnam College of Science & Technology, Daegu, Korea.
- KMID: 1503891
- DOI: http://doi.org/10.3349/ymj.2013.54.2.315
Abstract
- PURPOSE
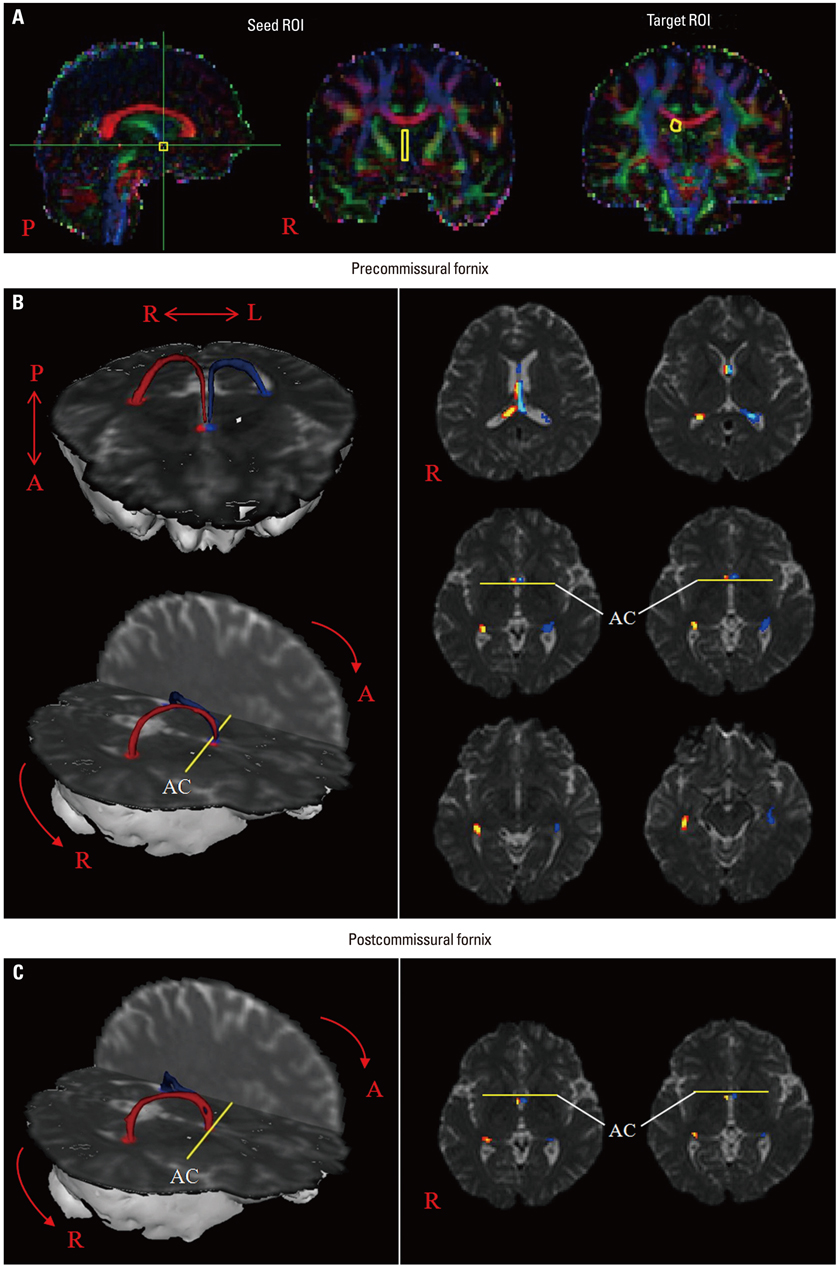
Other than a single case report, no diffusion tensor tractography (DTT) studies of the precommissural fornix in the human brain have been conducted. In the current study, we attempted to visualize the precommissural fornix in the human brain using DTT.
MATERIALS AND METHODS
We recruited 36 healthy volunteers for this study. Diffusion tensor images were scanned using a 1.5-T scanner, and the precommissural fornix was analyzed using Functional Magnetic Resonance Imaging of the Brain (FMRIB) software. Values of fractional anisotropy (FA), mean diffusivity (MD), and tract volume of the precommissural fornix were measured.
RESULTS
The precommissural fornix originated from the hippocampal formation on each hemisphere as a crus; both crura were then joined to the body of the fornix. The body of the fornix continued anteriorly to the level just superior to the anterior commissure, where it divided into each column of the precommissural fornix. Each column descended anteriorly to the anterior commissure and terminated in the septal nuclei. Values of FA, MD, and tract volumes of the precommissural fornix did not differ between the right and left hemispheres (p>0.05).
CONCLUSION
We believe that the methodology and results of this study would be helpful to future research on the precommissural fornix and in the elucidation of the pathology of diseases involving the precommissural fornix.
MeSH Terms
Figure
Reference
-
1. Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004. 46:71–117.2. Brisch R, Bernstein HG, Dobrowolny H, Krell D, Stauch R, Trübner K, et al. A morphometric analysis of the septal nuclei in schizophrenia and affective disorders: reduced neuronal density in the lateral septal nucleus in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011. 261:47–58.3. McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004. 28:285–305.
Article4. Henderson J, Greene E. Behavioral effects of lesions of precommissural and postcommissural fornix. Brain Res Bull. 1977. 2:123–129.
Article5. Thomas GJ. Delayed alternation in rats after pre- or postcommissural fornicotomy. J Comp Physiol Psychol. 1978. 92:1128–1136.
Article6. Park SA, Hahn JH, Kim JI, Na DL, Huh K. Memory deficits after bilateral anterior fornix infarction. Neurology. 2000. 54:1379–1382.
Article7. Poreh A, Winocur G, Moscovitch M, Backon M, Goshen E, Ram Z, et al. Anterograde and retrograde amnesia in a person with bilateral fornix lesions following removal of a colloid cyst. Neuropsychologia. 2006. 44:2241–2248.
Article8. Kuroki N, Kubicki M, Nestor PG, Salisbury DF, Park HJ, Levitt JJ, et al. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006. 60:22–31.
Article9. Yasuno F, Hirata M, Takimoto H, Taniguchi M, Nakagawa Y, Ikejiri Y, et al. Retrograde temporal order amnesia resulting from damage to the fornix. J Neurol Neurosurg Psychiatry. 1999. 67:102–105.
Article10. Korematsu K, Hori T, Morioka M, Kuratsu J. Memory impairment due to a small unilateral infarction of the fornix. Clin Neurol Neurosurg. 2010. 112:164–166.
Article11. Moudgil SS, Azzouz M, Al-Azzaz A, Haut M, Gutmann L. Amnesia due to fornix infarction. Stroke. 2000. 31:1418–1419.
Article12. Hong JH, Jang SH. Degeneration of cingulum and fornix in a patient with traumatic brain injury: diffuse tensor tractography study. J Rehabil Med. 2010. 42:979–981.
Article13. Chang MC, Kim SH, Kim OL, Bai DS, Jang SH. The relation between fornix injury and memory impairment in patients with diffuse axonal injury: a diffusion tensor imaging study. NeuroRehabilitation. 2010. 26:347–353.
Article14. Jang SH, Kim SH, Kim OL. Fornix injury in a patient with diffuse axonal injury. Arch Neurol. 2009. 66:1424–1425.
Article15. Sugiyama K, Kondo T, Higano S, Endo M, Watanabe H, Shindo K, et al. Diffusion tensor imaging fiber tractography for evaluating diffuse axonal injury. Brain Inj. 2007. 21:413–419.
Article16. Hong JH, Jang SH. Left fornical crus injury and verbal memory impairment in a patient with head trauma. Eur Neurol. 2010. 63:252.
Article17. Carpenter Malcolm B. Core Text of Neuroanatomy. 1991. 4th ed. Baltimore: Williams & Wilkins;373–375.18. Afifi AK, Bergman RA. Functional Neuroanatomy: Text and Atlas. 2005. 2nd ed. New York: McGraw-Hill;287–288.19. Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. 2008. 3rd ed. San Diego: Elsevier Academic Press;127. 129. 131. 133.20. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999. 45:265–269.
Article21. Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, et al. Disrupted integrity of the fornix is associated with impaired memory organization in schizophrenia. Schizophr Res. 2008. 103:52–61.
Article22. Zhuang L, Wen W, Trollor JN, Kochan NA, Reppermund S, Brodaty H, et al. Abnormalities of the Fornix in Mild Cognitive Impairment are Related to Episodic Memory Loss. J Alzheimers Dis. 2012. 29:629–639.
Article23. Rollins N. Semilobar holoprosencephaly seen with diffusion tensor imaging and fiber tracking. AJNR Am J Neuroradiol. 2005. 26:2148–2152.24. Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007. 34:144–155.
Article25. Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003. 6:750–757.
Article26. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004. 23:Suppl 1. S208–S219.
Article27. Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977. 172:49–84.
Article28. Watanabe T, Radulovic J, Spiess J, Natt O, Boretius S, Frahm J, et al. In vivo 3D MRI staining of the mouse hippocampal system using intracerebral injection of MnCl2. Neuroimage. 2004. 22:860–867.
Article29. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996. 111:209–219.
Article30. Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008. 34:51–61.
Article31. Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008. 27:1–7.
Article32. Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Quantitative diffusion tensor fiber tracking of age-related changes in the limbic system. Eur Radiol. 2008. 18:130–137.
Article33. Vann SD, Denby C, Love S, Montaldi D, Renowden S, Coakham HB. Memory loss resulting from fornix and septal damage: impaired supra-span recall but preserved recognition over a 24-hour delay. Neuropsychology. 2008. 22:658–668.
Article34. Lee SK, Kim DI, Kim J, Kim DJ, Kim HD, Kim DS, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics. 2005. 25:53–65.
Article35. Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005. 360:893–902.
Article36. Yamada K. Diffusion tensor tractography should be used with caution. Proc Natl Acad Sci U S A. 2009. 106:E14.
Article37. Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T. MR tractography: a review of its clinical applications. Magn Reson Med Sci. 2009. 8:165–174.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications
- Reversible Psychosis Caused by Disconnection of the Limbic System: Clinical Reasoning Using Diffusion Tensor Tractography
- Fornix Injury in a Patient with Rotavirus Encephalopathy: Diffusion Tensor Tractography Study
- The Nigrostriatal Tract between the Substantia Nigra and Striatum in the Human Brain: A Diffusion Tensor Tractography Study
- Diffusion Tensor Tractography of a Gliomatosis Cerebri: A Case Report