Korean J Physiol Pharmacol.
2012 Oct;16(5):333-337. 10.4196/kjpp.2012.16.5.333.
cAMP/PKA Agonist Restores the Fasting-Induced Down-Regulation of nNOS Expression in the Paraventricular Nucleus
- Affiliations
-
- 1Dental Research Institute, Department of Oral & Maxillofacial Surgery, Seoul National University School of Dentistry, Seoul 110-768, Korea. jwjahng@snu.ac.kr
- 2Department of Pharmacology, Wonkwang University School of Medicine, Iksan 570-749, Korea.
- 3Department of Family Practice and Community Health, Ajou University School of Medicine, Suwon 443-721, Korea.
- KMID: 1493966
- DOI: http://doi.org/10.4196/kjpp.2012.16.5.333
Abstract
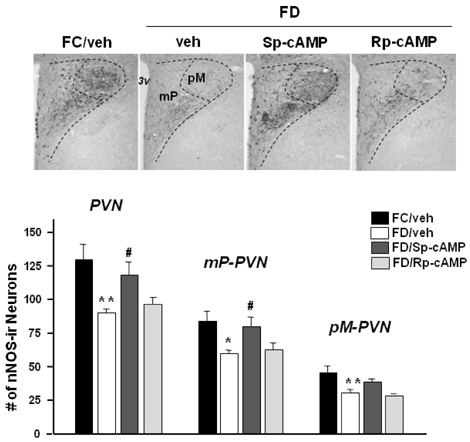
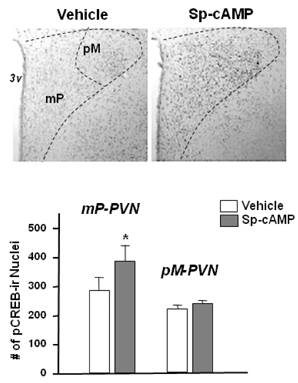
- Gene expression of neuronal nitric oxide synthase (nNOS) changes in the hypothalamic paraventricular nucleus (PVN) depending on feeding conditions, which is decreased during food deprivation and restored by refeeding, and phosphorylated cAMP response element binding protein (pCREB) was suggested to play a role in its regulation. This study was conducted to examine if the fasting-induced down-regulation of the PVN-nNOS expression is restored by activation of cAMP-dependent protein kinase A (cAMP/PKA) pathway. Freely moving rats received intracerebroventricular (icv) injection of cAMP/PKA activator Sp-cAMP (40 nmol) or vehicle (sterilized saline) following 48 h of food deprivation. One hour after drug injections, rats were transcardially perfused with 4% paraformaldehyde, and the PVN tissues were processed for nNOS or pCREB immunohistochemistry. Sp-cAMP significantly increased not only nNOS but also pCREB immunoreactivities in the PVN of food deprived rats. Fasting-induced down-regulation of the PVN-nNOS was restored by 1 h after the icv Sp-cAMP. Results suggest that cAMP/PKA pathway may mediate the regulation of the PVN-nNOS expression depending on different feeding conditions.
Keyword
MeSH Terms
-
Animals
Cyclic AMP Response Element-Binding Protein
Cyclic AMP-Dependent Protein Kinases
Down-Regulation
Food Deprivation
Formaldehyde
Gene Expression
Immunohistochemistry
Nitric Oxide Synthase Type I
Paraventricular Hypothalamic Nucleus
Polymers
Rats
Cyclic AMP Response Element-Binding Protein
Cyclic AMP-Dependent Protein Kinases
Formaldehyde
Nitric Oxide Synthase Type I
Polymers
Figure
Cited by 1 articles
-
Predominant D1 Receptors Involvement in the Over-expression of CART Peptides after Repeated Cocaine Administration
Zhenzhen Hu, Eun-Hye Oh, Yeon Bok Chung, Jin Tae Hong, Ki-Wan Oh
Korean J Physiol Pharmacol. 2015;19(2):89-97. doi: 10.4196/kjpp.2015.19.2.89.
Reference
-
1. Ueta Y, Levy A, Chowdrey HS, Lightman SL. Inhibition of hypothalamic nitric oxide synthase gene expression in the rat paraventricular nucleus by food deprivation is independent of serotonin depletion. J Neuroendocrinol. 1995. 7:861–865.2. O'Shea RD, Gundlach AL. Food or water deprivation modulate nitric oxide synthase (NOS) activity and gene expression in rat hypothalamic neurones: correlation with neurosecretory activity? J Neuroendocrinol. 1996. 8:417–425.3. Kim YM, Lee JY, Choi SH, Kim DG, Jahng JW. RU486 blocks fasting-induced decrease of neuronal nitric oxide synthase in the rat paraventricular nucleus. Brain Res. 2004. 1018:221–226.4. Jahng JW, Lee JH, Kim GT, Kim YM, Houpt TA, Kim DG. Adrenalectomy abolishes fasting-induced down-regulation of NADPH-diaphorase in the rat paraventricular nucleus. Yonsei Med J. 2004. 45:123–128.5. Jahng JW, Lee JY, Yoo SB, Kim YM, Ryu V, Kang DW, Lee JH. Refeeding-induced expression of neuronal nitric oxide synthase in the rat paraventricular nucleus. Brain Res. 2005. 1048:185–192.6. van Haasteren GA, Linkels E, van Toor H, Klootwijk W, Kaptein E, de Jong FH, Reymond MJ, Visser TJ, de Greef WJ. Effects of long-term food reduction on the hypothalamus-pituitary-thyroid axis in male and female rats. J Endocrinol. 1996. 150:169–178.7. Yoshihara T, Honma S, Katsuno Y, Honma K. Dissociation of paraventricular NPY release and plasma corticosterone levels in rats under food deprivation. Am J Physiol. 1996. 271:E239–E245.8. Lee JY, Kang DW, Kim DG, Jahng JW. Fasting-induced down-regulation of NADPH-diaphorase in the magnocellular PVN of rats. Yonsei Med J. 2004. 45:917–922.9. Jeong Y, Won J, Kim C, Yim J. 5'-Flanking sequence and promoter activity of the rabbit neuronal nitric oxide synthase (nNOS) gene. Mol Cells. 2000. 10:566–574.10. Rife TK, Xie J, Redman C, Young AP. The 5'2 promoter of the neuronal nitric oxide synthase dual promoter complex mediates inducibility by nerve growth factor. Brain Res Mol Brain Res. 2000. 75:225–236.11. Sasaki M, Gonzalez-Zulueta M, Huang H, Herring WJ, Ahn S, Ginty DD, Dawson VL, Dawson TM. Dynamic regulation of neuronal NO synthase transcription by calcium influx through a CREB family transcription factor-dependent mechanism. Proc Natl Acad Sci USA. 2000. 97:8617–8622.12. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989. 59:675–680.13. Son GH, Geum D, Jung H, Kim K. Glucocorticoid inhibits growth factor-induced differentiation of hippocampal progenitor HiB5 cells. J Neurochem. 2001. 79:1013–1021.14. Lee JY, Lee JH, Kim DG, Jahng JW. Dexamethasone blocks the refeeding-induced phosphorylation of cAMP response element-binding protein in the rat hypothalamus. Neurosci Lett. 2003. 344:107–111.15. Timofeeva E, Picard F, Duclos M, Deshaies Y, Richard D. Neuronal activation and corticotropin-releasing hormone expression in the brain of obese (fa/fa) and lean (fa/?) Zucker rats in response to refeeding. Eur J Neurosci. 2002. 15:1013–1029.16. Sheriff S, Chance WT, Iqbal S, Rizvi TA, Xiao C, Kasckow JW, Balasubramaniam A. Hypothalamic administration of cAMP agonist/PKA activator inhibits both schedule feeding and NPY-induced feeding in rats. Peptides. 2003. 24:245–254.17. Jahng JW, Spencer CM, Choi SH, Kim DG, Houpt TA. Nitric oxide is involved in lithium-induced immediate early gene expressions in the adrenal medulla. Eur J Pharmacol. 2004. 489:111–116.18. Spencer CM, Jahng JW, Ryu V, Houpt TA. Lithium-induced gene expression of inducible cyclic adenosine monophosphate early repressor in the rat adrenal gland. J Neurosci Res. 2005. 82:273–282.19. Lee JH, Cha MJ, Yoo SB, Moon YW, Noh SJ, Jahng JW. Leptin blocks the fasting-induced increase of pERK1/2 in the paraventricular nucleus of rats. Regul Pept. 2010. 162:122–128.20. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1986. New York: Academic Press.21. Jahng JW, Houpt TA, Kim SJ, Joh TH, Son JH. Neuropeptide Y mRNA and serotonin innervation in the arcuate nucleus of anorexia mutant mice. Brain Res. 1998. 790:67–73.22. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996. 382:250–252.23. Boissel JP, Bros M, Schröck A, Gödtel-Armbrust U, Förstermann U. Cyclic AMP-mediated upregulation of the expression of neuronal NO synthase in human A673 neuroepithelioma cells results in a decrease in the level of bioactive NO production: analysis of the signaling mechanisms that are involved. Biochemistry. 2004. 43:7197–7206.24. Eberhardt W, Engels C, Müller R, Pfeilschifter J. Mechanisms of dexamethasone-mediated inhibition of cAMP-induced tPA expression in rat mesangial cells. Kidney Int. 2002. 62:809–821.25. Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Døskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3',5'-cyclic phosphorothioates. J Biol Chem. 1990. 265:10484–10491.26. Wu G, Meininger CJ. Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr. 2002. 22:61–86.27. Park SH, Lee YJ, Lim MJ, Kim EJ, Lee JH, Han HJ. High glucose inhibits fructose uptake in renal proximal tubule cells: involvement of cAMP, PLC/PKC, p44/42 MAPK, and cPLA2. J Cell Physiol. 2004. 200:407–416.28. Santangelo GM. Glucose signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2006. 70:253–282.29. Otsubo H, Kondoh T, Shibata M, Torii K, Ueta Y. Induction of Fos expression in the rat forebrain after intragastric administration of monosodium L-glutamate, glucose and NaCl. Neuroscience. 2011. 196:97–103.30. Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992. 8:3–11.31. Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995. 57:683–706.32. Yun HY, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol. 1996. 10:291–316.33. Boissel JP, Schwarz PM, Förstermann U. Neuronal-type NO synthase: transcript diversity and expressional regulation. Nitric Oxide. 1998. 2:337–349.34. Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994. 14:5147–5159.35. Förstermann U, Boissel JP, Kleinert H. Expressional control of the 'constitutive' isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J. 1998. 12:773–790.36. Casto RM, VanNess JM, Overton JM. Effects of central leptin administration on blood pressure in normotensive rats. Neurosci Lett. 1998. 246:29–32.37. Krukoff TL. Central actions of nitric oxide in regulation of autonomic functions. Brain Res Brain Res Rev. 1999. 30:52–65.38. Stern JE, Li Y, Zhang W. Nitric oxide: a local signalling molecule controlling the activity of pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Acta Physiol Scand. 2003. 177:37–42.39. Kadowaki K, Kishimoto J, Leng G, Emson PC. Up-regulation of nitric oxide synthase (NOS) gene expression together with NOS activity in the rat hypothalamo-hypophysial system after chronic salt loading: evidence of a neuromodulatory role of nitric oxide in arginine vasopressin and oxytocin secretion. Endocrinology. 1994. 134:1011–1017.40. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983. 6:269–324.41. Burlet AJ, Jhanwar-Uniyal M, Chapleur-Chateau M, Burlet CR, Leibowitz SF. Effect of food deprivation and refeeding on the concentration of vasopressin and oxytocin in discrete hypothalamic sites. Pharmacol Biochem Behav. 1992. 43:897–905.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fasting-induced Down-regulation of NADPH-diaphorase in the Magnocellular PVN of Rats
- Adrenalectomy Abolishes Fasting-induced Down-regulation of NADPH-diaphorase in the Rat Paraventricular Nucleus
- Predominant D1 Receptors Involvement in the Over-expression of CART Peptides after Repeated Cocaine Administration
- Effect of Chronic Alcohol Intake on Vasopressin and Oxytocin-containing Neurons in the Paraventricular and Supraoptic Nucleus of the Rat Hypothalamus
- Adaptation of cAMP signaling system in SH-SY5Y neuroblastoma cells following expression of a constitutively active stimulatory G protein alpha, Q227L Gsalpha