J Korean Bone Joint Tumor Soc.
2012 Jun;18(1):20-27. 10.5292/jkbjts.2012.18.1.20.
Differential Expression of CXCR4 in Conventional High-grade and Low-grade Central Osteosarcoma and Its Prognostic Implications
- Affiliations
-
- 1Department of Pathology, Hallym University College of Medicine, Anyang, Korea.
- 2Servizio di Anatomia Pathologica, Istituto Ortopedico Rizzoli, Bologna, Italy.
- 3Department of Pathology, Kyung Hee University College of Medicine, Seoul, Korea. ykpark@khmc.or.kr
- KMID: 1438403
- DOI: http://doi.org/10.5292/jkbjts.2012.18.1.20
Abstract
- PURPOSE
The chemokine receptor CXCR4 has been reported to be aberrantly expressed in human cancer and has been shown to participate in cancer metastasis. We compared the expression of CXCR4 in conventional high-grade and low-grade central osteosarcomas, and determined if an association between CXCR4 expression and prognosis could be made.
MATERIALS AND METHODS
We performed the immunohistochemistry for CXCR4 in a total of 63 patients with osteosarcoma and determined the relationships according to the clinicopathologic variables and overall survival rates.
RESULTS
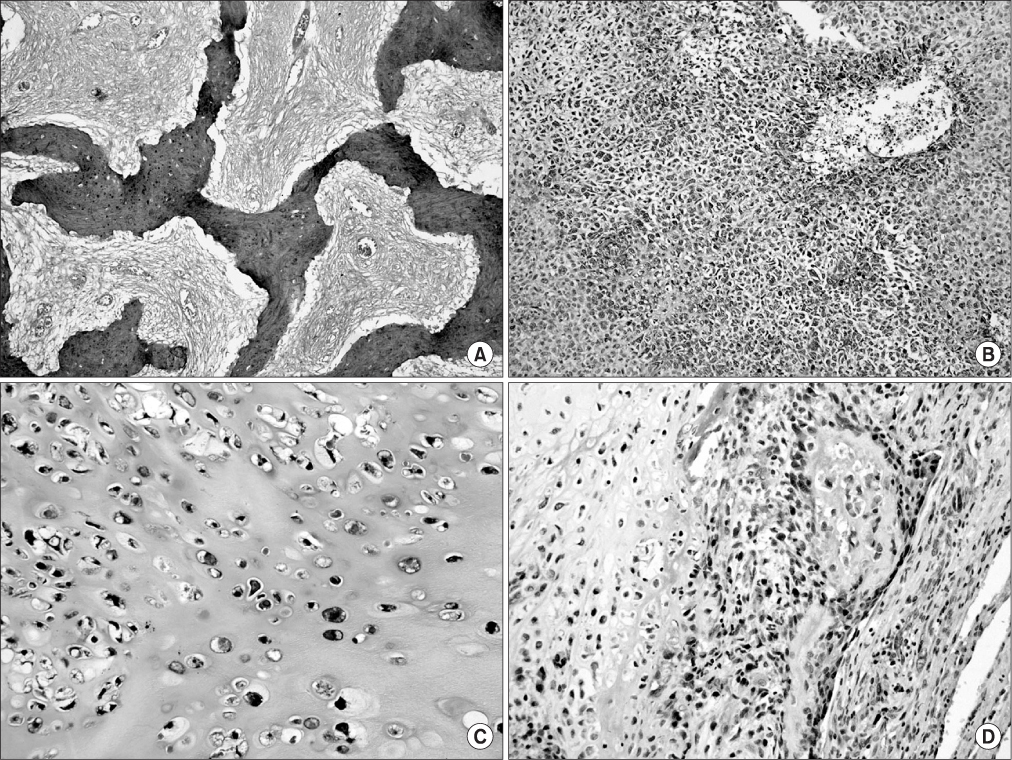
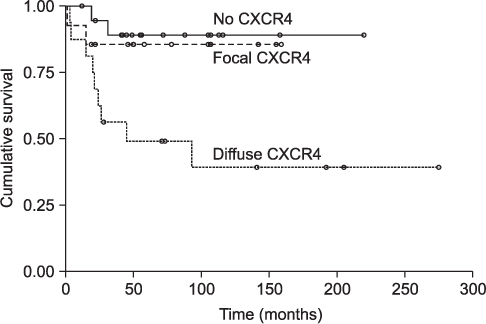
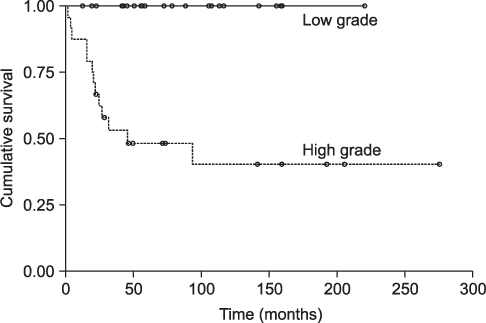
CXCR4 was detected in 76.3% of conventional high-grade osteosarcoma patients and in 36% of low-grade central osteosarcomas. Diffuse expression was noted in 47.4% of the high-grade osteosarcomas and all low-grade cases were focal positive. CXCR4 expression was significantly correlated with histologic grade (p<0.0001). While overall survival rate was reduced significantly with increased CXCR4 expression (p=0.0058), higher histologic grade (p<0.0001), and younger age (p=0.0140), survival rate did not correlate with gender, tumor size, or AJCC stage.
CONCLUSION
Our results suggest that CXCR4 expression is associated with higher-grade tumors and with poor prognosis for osteosarcoma patients.
Keyword
Figure
Reference
-
1. Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokines grammar for immune cells. Annu Rev Immunol. 2004. 22:891–928.2. Oda Y, Yamamoto H, Tamiya S, et al. CXCR4 and VEGF expression in the primary site and the metastatic site of human osteosarcoma: analysis within a group of patients, all of whom developed lung metastasis. Mod Pathol. 2006. 19:738–745.3. Chinni SR, Yamamoto H, Dong Z, Sabbota A, Bonfil RD, Cher ML. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol Cancer Res. 2008. 6:446–457.4. Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001. 410:50–56.5. Spano JP, Andre F, Morat L, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004. 15:613–617.6. Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005. 11:1835–1841.7. Koishi K, Yoshikawa R, Tsujimura T, et al. Persistent CXCR4 expression after preoperative chemoradiotherapy predicts early recurrence and poor prognosis in esophageal cancer. World J Gastroenterol. 2006. 12:7585–7590.8. Jiang YP, Wu XH, Shi B, Wu WX, Yin GR. Expression of chemokine CXCL12 and its receptor CXCR4 in human epithelial ovarian cancer: an independent prognostic factor for tumor progression. Gynecol Oncol. 2006. 103:226–233.9. Azenshtein E, Luboshits G, Shina S, et al. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002. 62:1093–1102.10. Fan TM, Barger AM, Fredrickson RL, Fitzsimmons D, Garrett LD. Investigating CXCR4 expression in canine appendicular osteosarcoma. J Vet Intern Med. 2008. 22:602–608.11. Laverdiere C, Hoang BH, Yang R, et al. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res. 2005. 11:2561–2567.12. Perissinotto E, Cavalloni G, Leone F, et al. Involvement of chemokine receptor 4/stromal cell-derived factor 1 system during osteosarcoma tumor progression. Clin Cancer Res. 2005. 11:490–497.13. Unni KK, Dahlin DC. Grading of bone tumors. Semin Diagn Pathol. 1984. 1:165–172.14. Paoletti S, Borzi RM, Mazzetti I, et al. Human osteosarcoma cells release matrix degrading enzymes in response to chemokine activation. Int J Oncol. 2001. 18:11–16.15. Libura J, Drukala J, Majka M, et al. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002. 100:2597–2606.16. Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002. 62:7328–7334.17. Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004. 64:8604–8612.18. Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002. 62:1832–1837.19. Bai S, Wang D, Klein MJ, Siegal GP. Characterization of CXCR4 expression in chondrosarcoma of bone. Arch Pathol Lab Med. 2011. 135:753–758.20. de Nigris F, Rossiello R, Schiano C, et al. Deletion of Yin Yang 1 protein in osteosarcoma cells on cell invasion and CXCR4/angiogenesis and metastasis. Cancer Res. 2008. 68:1797–1808.21. Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002. 62:7203–7206.22. Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004. 14:171–179.23. Gobert M, Treilleux I, Bendriss-Vermare N, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009. 69:2000–2009.24. von Luettichau I, Nathrath M, Burdach S, Huss R, Segerer S, Nelson PJ. Mononuclear infiltrates in osteosarcoma and chemokine receptor expression. Clin Cancer Res. 2006. 12:5253–5254.25. Takenaga M, Tamamura H, Hiramatsu K, et al. A single treatment with microcapsules containing a CXCR4 antagonist suppresses pulmonary metastasis of murine melanoma. Biochem Biophys Res Commun. 2004. 320:226–232.26. Kim SY, Lee CH, Midura BV, et al. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2008. 25:201–211.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Carbonic Anhydrase IX Correlates with Histologic Grade and Metastasis in Osteosarcoma
- Overexpression of Metastatic Tumor Antigen in Osteosarcoma: Comparison between Conventional High-Grade and Central Low-Grade Osteosarcoma
- Low-grade Osteosarcoma of the Spine: A Case Report
- Osteosarcoma arising on the surface of long bones: a review of radiologic and histologic spectrum in 9 cases
- Correlation between Chemokine Receptor CXCR4 Expression and Prognostic Factors in Patients with Prostate Cancer