J Nutr Health.
2016 Aug;49(4):223-232. 10.4163/jnh.2016.49.4.223.
Validation of G-protein beta-3 subunit gene C825T polymorphism as predictor of obesogenic epidemics in overweight/obese Korean children
- Affiliations
-
- 1Department of Food Science and Nutrition, Jeju National University, Jeju 63243, Korea.
- 2Imported Food Analysis Team, Ministry of Food Drug Safety, Gyeongin Regional, Food & Drug Administration, Gyeonggi 13809, Korea.
- 3Department of Food and Nutrition and Research Institute of Obesity Sciences, Sungshin Women's university, Seoul 01133, Korea. mlee@sungshin.ac.kr
- KMID: 2351350
- DOI: http://doi.org/10.4163/jnh.2016.49.4.223
Abstract
- PURPOSE
We investigated the potential interaction between the G-protein beta-3 subunit gene (GNB3) C825T polymorphism, a risk factor for chronic disease in various ethnicities, and obesogenic environments in overweight/obese Korean children.
METHODS
The present study was conducted as a cross-sectional study using measures of anthropometry, blood pressure (BP), and fasting blood samples as well as 3-day food records. Subjects were recruited from seven elementary schools in an urban district in Seoul, South Korea, between 2007 and 2008. A total of 1,260 children aged 8-9 years were recruited in the study, including 633 boys (50.3%) and 627 girls (49.7%).
RESULTS
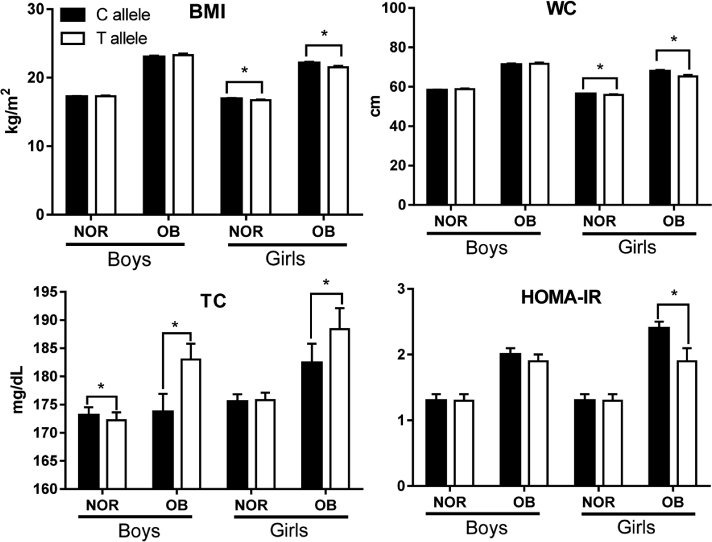
The allele frequencies of the GNB3 polymorphism were C allele = 49.7% and T allele = 50.3% in subjects. In general, boys with T allele had higher BMI, systolic BP (SBP), and triglycerides, although their energy intake was not significantly different from boys with C allele. In contrast to boys, girls with T allele had lower BMI but higher SBP and energy intake than those with C allele. The girls with T allele had a significantly lower BMI and waist circumference in both the normal weight group and obese group (OB). T allele carriers in both genders had significantly higher TC than C allele carriers in the OB group. At last, girls with T allele in OB appeared to have significantly lower HOMA-IR than those with C allele.
CONCLUSION
Unlike higher risk for negative health outcomes by the GNB3 polymorphism in various ethnicities, GNB3 polymorphism did not influence obesogenic environments in overweight/obese Korean children.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Salt-sensitive genes and their relation to obesity
∗
Yong-Pil Cheon, Myoungsook Lee
J Nutr Health. 2017;50(3):217-224. doi: 10.4163/jnh.2017.50.3.217.
Reference
-
1. Moon JS. Secular trends of body sizes in Korean children and adolescents: from 1965 to 2010. Korean J Pediatr. 2011; 54(11):436–442.
Article2. Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJ. Health consequences of obesity. Arch Dis Child. 2003; 88(9):748–752.
Article3. Bouchard C. Current understanding of the etiology of obesity: genetic and nongenetic factors. Am J Clin Nutr. 1991; 53:6 Suppl. 1561S–1565S.
Article4. Comuzzie AG, Allison DB. The search for human obesity genes. Science. 1998; 280(5368):1374–1377.
Article5. Alfredo Martínez J, Enríquez L, Moreno-Aliaga MJ, Martí A. Genetics of obesity. Public Health Nutr. 2007; 10(10A):1138–1144.6. Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998; 273(2):669–672.
Article7. Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008; 9(1):60–71.
Article8. Tesmer JJ. The quest to understand heterotrimeric G protein signaling. Nat Struct Mol Biol. 2010; 17(6):650–652.
Article9. Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer's disease. Nat Rev Neurosci. 2011; 12(2):73–87.
Article10. Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochim Biophys Acta. 2010; 1802(12):1268–1275.
Article11. Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011; 10(1):47–60.
Article12. Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011; 7(6):362–372.
Article13. Rosskopf D, Busch S, Manthey I, Siffert W. G protein beta 3 gene: structure, promoter, and additional polymorphisms. Hypertension. 2000; 36(1):33–41.14. Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B. Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet. 1998; 18(1):45–48.15. Siffert W, Naber C, Walla M, Ritz E. G protein β3 subunit 825T allele and its potential association with obesity in hypertensive individuals. J Hypertens. 1999; 17(8):1095–1098.
Article16. Klenke S, Kussmann M, Siffert W. The GNB3 C825T polymorphism as a pharmacogenetic marker in the treatment of hypertension, obesity, and depression. Pharmacogenet Genomics. 2011; 21(9):594–606.
Article17. Hegele RA, Harris SB, Hanley AJ, Cao H, Zinman B. G protein beta3 subunit gene variant and blood pressure variation in Canadian Oji-Cree. Hypertension. 1998; 32(4):688–692.18. Siffert W, Rosskopf D, Erbel R. Genetic polymorphism of the Gprotein beta3 subunit, obesity and essential hypertension. Herz. 2000; 25(1):26–33.19. Poch E, Giner V, González-Núñez D, Coll E, Oriola J, de la Sierra A. Association of the G protein beta3 subunit T allele with insulin resistance in essential hypertension. Clin Exp Hypertens. 2002; 24(5):345–353.20. Brand E, Wang JG, Herrmann SM, Staessen JA. An epidemiological study of blood pressure and metabolic phenotypes in relation to the Gbeta3 C825T polymorphism. J Hypertens. 2003; 21(4):729–737.21. Siffert W, Forster P, Jöckel KH, Mvere DA, Brinkmann B, Naber C, Crookes R, Du P, Epplen JT, Fridey J, Freedman BI, Müller N, Stolke D, Sharma AM, Al Moutaery K, Grosse-Wilde H, Buerbaum B, Ehrlich T, Ahmad HR, Horsthemke B, Du Toit ED, Tiilikainen A, Ge J, Wang Y, Rosskopf D, Rosskopf D. Worldwide ethnic distribution of the G protein beta3 subunit 825T allele and its association with obesity in Caucasian, Chinese, and Black African individuals. J Am Soc Nephrol. 1999; 10(9):1921–1930.22. Li B, Ge D, Wang Y, Zhao W, Zhou X, Gu D, Chen R. G protein β3 subunit gene variants and essential hypertension in the northern Chinese Han population. Ann Hum Genet. 2005; 69(Pt 4):468–473.
Article23. Bae Y, Park C, Han J, Hong YJ, Song HH, Shin ES, Lee JE, Han BG, Jang Y, Shin DJ, Yoon SK. Interaction between GNB3 C825T and ACE I/D polymorphisms in essential hypertension in Koreans. J Hum Hypertens. 2007; 21(2):159–166.
Article24. Ko KD, Kim KK, Suh HS, Hwang IC. Associations between the GNB3 C825T polymorphism and obesity-related metabolic risk factors in Korean obese women. J Endocrinol Invest. 2014; 37(11):1117–1120.
Article25. Hwang IC, Kim KK, Ahn HY, Suh HS, Oh SW. Effect of the Gprotein beta3 subunit 825T allele on the change of body adiposity in obese female. Diabetes Obes Metab. 2013; 15(3):284–286.26. Babu A, Fogelfeld L. Metabolic syndrome and prediabetes. Dis Mon. 2006; 52(2-3):55–144.
Article27. Vekic J, Topic A, Zeljkovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. LDL and HDL subclasses and their relationship with Framingham risk score in middle-aged Serbian population. Clin Biochem. 2007; 40(5-6):310–316.
Article28. Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011; 17(10):594–603.
Article29. Lamarche B, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 1997; 17(6):1098–1105.30. Lee M, Jang Y, Kim K, Cho H, Jee SH, Park Y, Kim MK. Relationship between HDL3 subclasses and waist circumferences on the prevalence of metabolic syndrome: KMSRI-Seoul Study. Atherosclerosis. 2010; 213(1):288–293.
Article31. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, Kim YT, Lee CG. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008; 51(1):1–25.
Article32. Seo J, Cho Y, Kang J, Hur Y, Park H, Kim K, Kwon S. New diagnostic criteria for obesity and overweight in Korean children and adolescents using 2007 Korean National Growth Charts. Obes Res Clin Pract. 2013; 7(3):e182–e189.
Article33. Lee M, Kim MK, Kim SM, Park H, Park CG, Park HK. Genderbased differences on the association between salt-sensitive genes and obesity in Korean children aged between 8 and 9 years. PLoS One. 2015; 10(3):e0120111.
Article34. Pérusse M, Pascot A, Després JP, Couillard C, Lamarche B. A new method for HDL particle sizing by polyacrylamide gradient gel electrophoresis using whole plasma. J Lipid Res. 2001; 42(8):1331–1334.
Article35. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997; 65:4 Suppl. 1220S–1228S.
Article36. Frey UH, Moebus S, Möhlenkamp S, Kälsch H, Bauer M, Lehmann N, Nöthen M, Mühleisen TW, Stang A, Erbel R, Jöckel KH, Peters J, Siffert W. GNB3 gene 825 TT variant predicts hard coronary events in the population-based Heinz Nixdorf Recall study. Atherosclerosis. 2014; 237(2):437–442.
Article37. Garcés C, Gutierrez-Guisado J, Benavente M, Cano B, Viturro E, Ortega H, de Oya M. Obesity in Spanish schoolchildren: relationship with lipid profile and insulin resistance. Obes Res. 2005; 13(6):959–963.
Article38. Numata M. High density lipoprotein particle size in children: relation to atherogenic dyslipidemia. Clin Pediatr Endocrinol. 2004; 13(1):1–9.
Article39. Hegele RA, Anderson C, Young TK, Connelly PW. G-protein beta3 subunit gene splice variant and body fat distribution in Nunavut Inuit. Genome Res. 1999; 9(10):972–977.40. Benjafield AV, Lin RC, Dalziel B, Gosby AK, Caterson ID, Morris BJ. G-protein beta3 subunit gene splice variant in obesity and overweight. Int J Obes Relat Metab Disord. 2001; 25(6):777–780.41. Rankinen T, Rice T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bouchard C. G protein beta 3 polymorphism and hemodynamic and body composition phenotypes in the HERITAGE Family Study. Physiol Genomics. 2002; 8(2):151–157.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation of G-protein beta-3 subunit gene C825T polymorphism as predictor of obesogenic epidemics in overweight/obese Korean children
- G-Protein Beta3 Subunit C825T Polymorphism in Patients With Overlap Syndrome of Functional Dyspepsia and Irritable Bowel Syndrome
- Serotonin Transporter Gene and G-protein beta3 C825T Gene Polymorphism in Patients with Functional Dyspepsia and Irritable Bowel Syndrome
- Polymorphisms of the Serotonin Transporter Gene and G-Protein beta3 Subunit Gene in Korean Children with Irritable Bowel Syndrome and Functional Dyspepsia
- Association of G-Protein β3 Subunit C825T Polymorphism with Seasonal Variations in Mood and Behavior