J Nutr Health.
2016 Jun;49(3):135-143. 10.4163/jnh.2016.49.3.135.
Suppressive effects of ethanol extract of Aralia elata on UVB-induced oxidative stress in human keratinocytes
- Affiliations
-
- 1Institute on Aging, Seoul National University College of Medicine, Seoul 03080, Korea. kwakcs@snu.ac.kr
- KMID: 2328178
- DOI: http://doi.org/10.4163/jnh.2016.49.3.135
Abstract
- PURPOSE
Ultraviolet (UV)-induced oxidative stress contributes to several adverse biological effects on skin. Many phenolic phytochemicals have been shown to have antioxidant properties and protect skin cells from UV-induced oxidative damage. In this study, we investigated whether or not Aralia elata (AE) has a protective effect against UVB-induced reactive oxygen species (ROS), ultimately leading to photoaging.
METHODS
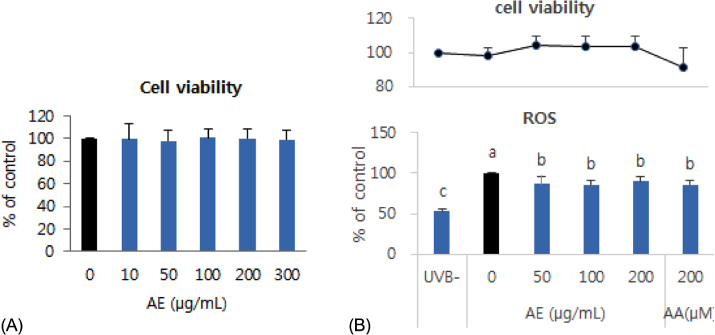
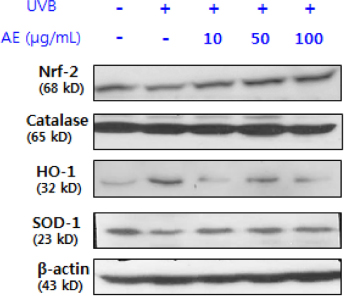
Phenolic content of dried AE and antioxidant properties of AE extract in 70% ethanol weredetermined by measuring DPPH and ABTS radical scavenging activities and ferric reducing antioxidant power (FRAP). The effect of AE extract on cellular ROS generation and expression levels of oxidative stress-response proteins such as superoxide dismutase (SOD)-1, catalase, nuclear factor-erythroid 2-related factor (Nrf)-2,and heme oxygenase (HO)-1 in UVB-irradiated (75 mJ/cm²) human keratinocytes (HaCaT) were further determined by 2'-7'-dichlorofluoresceine diacetate assay and Western blotting, respectively.
RESULTS
The total phenolic and flavonoid contents of dried AE were 20.15 mg tannic acid/g and 18.75 mg rutin/g, respectively. The ICâ‚…â‚€ of AE extract against DPPH radical was 98.5 µg/mL, and ABTS radical scavenging activity and FRAP upon treatment with 1,000 µg/mL of AE extract were 41.8 µg ascorbic acid (AA) eq./mL and 29.7 µg AA eq./mL,m respectively. Pretreatment with AE extract significantly reduced (p < 0.05) ROS generation compared to that in UVB-irradiated control HaCaT cells. Pretreatment with AE extract reversed reduction of Nrf-2 and SOD-1 protein expression and induction of HO-1 protein expression caused by UVB exposure in HaCaT cells, whereas it did not affect catalase expression.
CONCLUSION
AE extract in 70% ethanol demonstrated a protective effect against UVB-induced oxidative stress and decreased expression of Nrf-2 and SOD-1 in human keratinocytes. These findings suggest that AE ethanol extract might have potential as a natural resource for a skin antiphotoaging product in the food and cosmetic industry.
Keyword
MeSH Terms
-
Aralia*
Ascorbic Acid
Blotting, Western
Catalase
Ethanol*
Heme Oxygenase (Decyclizing)
Humans*
Keratinocytes*
Natural Resources
Oxidative Stress*
Phenol
Phytochemicals
Reactive Oxygen Species
Skin
Superoxide Dismutase
Ascorbic Acid
Catalase
Ethanol
Heme Oxygenase (Decyclizing)
Phenol
Reactive Oxygen Species
Superoxide Dismutase
Figure
Cited by 1 articles
-
Inhibitory effect of Aralia elata ethanol extract against skin damage in UVB-exposed human keratinocytes and human dermal fibroblasts
Jiwon Yang, Chungshil Kwak
J Nutr Health. 2016;49(6):429-436. doi: 10.4163/jnh.2016.49.6.429.
Reference
-
1. Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002; 123(7):801–810.
Article2. Yoo HG, Lee BH, Kim W, Lee JS, Kim GH, Chun OK, Koo SI, Kim DO. Lithospermum erythrorhizon extract protects keratinocytes and fibroblasts against oxidative stress. J Med Food. 2014; 17(11):1189–1196.
Article3. Debacq-Chainiaux F, Leduc C, Verbeke A, Toussaint O. UV, stress and aging. Dermatoendocrinol. 2012; 4(3):236–240.
Article4. Kim M, Park YG, Lee HJ, Lim SJ, Nho CW. Youngiasides A and C isolated from Youngia denticulatum inhibit UVB-indeced MMP expression and promote type 1 procollagen production via repression of MAPK/AP-1/NF-kB and activation of AMPK/Nrf2 in HaCat cells and human dermal fibroblasts. J Agric Food Chem. 2015; 63(22):5428–5438.5. Fernández-García E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014; 5(9):1994–2003.
Article6. Offord EA, Gautier JC, Avanti O, Scaletta C, Runge F, Krämer K, Applegate LA. Photoprotective potential of lycopene, beta-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic Biol Med. 2002; 32(12):1293–1303.7. Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol. 2012; 67(5):1013–1024.
Article8. Marrot L, Jones C, Perez P, Meunier JR. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008; 21(1):79–88.9. Patwardhan J, Bhatt P. Ultraviolet-B protective effect of flavonoids from Eugenia caryophylata on human dermal fibroblast cells. Pharmacogn Mag. 2015; 11:Suppl 3. S397–S406.10. Hwang E, Lee DG, Park SH, Oh MS, Kim SY. Coriander leaf extract exerts antioxidant activity and protects against UVB-induced photoaging of skin by regulation of procollagen type I and MMP-1 expression. J Med Food. 2014; 17(9):985–995.
Article11. Wang M, Xu X, Xu H, Wen F, Zhang X, Sun H, Yao F, Sun G, Sun X. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J Ethnopharmacol. 2014; 155(1):240–247.
Article12. Shin KH, Cho SY, Lee MK, Lee JS, Kim MJ. Effects of Aralia elata, Acanthopanacis cortex and Ulmus davidiana water extracts on plasma biomarkers in streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2004; 33(9):1457–1462.13. Kim YH, Im JG. Effect of saponin from the shoot of Aralia elata in normal rats and streptozotocin induced diabetic rats. J Korean Soc Food Sci Nutr. 1999; 28(44):912–916.14. Suh SJ, Jin UH, Kim KW, Son JK, Lee SH, Son KH, Chang HW, Lee YC, Kim CH. Triterpenoid saponin, oleanolic acid 3-O-beta-d-glucopyranosyl(1->3)-α-L-rhamnopyranosyl(1->2)-α-L-arabinopyranoside (OA) from Aralia elata inhibits LPS-induced nitric oxide production by down-regulated NF-kB in raw 264.7 cells. Arch Biochem Biophys. 2007; 467(2):227–233.15. Lee JH, Jeong CS. Suppressive effects on the biosynthesis of inflammatory mediators by Aralia elata extract fractions in macrophage cells. Environ Toxicol Pharmacol. 2009; 28(3):333–341.
Article16. Cha JY, Ahn HY, Eom KE, Park BK, Jun BS, Cho YS. Antioxidative activity of Aralia elata shoot and leaf extracts. J Life Sci. 2009; 19(5):652–658.17. Tomatsu M, Ohnishi-Kameyama M, Shibamoto N. Aralin, a new cytotoxic protein from Aralia elata, inducing apoptosis in human cancer cells. Cancer Lett. 2003; 199(1):19–25.
Article18. Chung CK, Jung ME. Ethanol fraction of Aralia elata Seemann enhances antioxidant activity and lowers serum lipids in rats when administered with Benzo(α)pyrene. Biol Pharm Bull. 2003; 26(10):1502–1504.19. Chae SK, Kang GS, Ma SJ, Bang KW, Oh MW, Oh SH. Determination of flavonoid contnet in citrus. Standard Food Analysis. Seoul: Jigumoonwhasa;2002. p. 581–582.20. Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999; 299:152–178.21. Senba Y, Nishishita T, Saito K, Yoshioka H, Yoshioka H. Stopped-flow and spectrophotometric study on radical scavenging by tea catechins and model compound. Chem Pharm Bull (Tokyo). 1999; 47(10):1369–1374.22. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10):1231–1237.
Article23. Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001; 49(8):4083–4089.24. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2):55–63.
Article25. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976; 72(1-2):248–254.
Article26. Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997; 2(4):152–159.
Article27. Nakayama T, Niimi T, Osawa T, Kawakishi S. The protective role of polyphenols in cytotoxicity of hydrogen peroxide. Mutat Res. 1992; 281(2):77–80.
Article28. Toda M, Okubo S, Hiyoshi R, Shimamura T. The bactericidal activity of tea and coffee. Lett Appl Microbiol. 1989; 8(4):123–125.
Article29. Middleton E Jr, Kandaswami CC. Potential health-promoting properties of citrus flavonoids. Food Technol. 1994; 48(11):115–119.30. Kang MH, Park CG, Cha MS, Seong NS, Chung HK, Lee JB. Component characteristics of each extract prepared by different extract methods from by-products of Glycyrrhizia uralensis. J Korean Soc Food Sci Nutr. 2001; 30(1):138–142.31. Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001; 49(7):3420–3424.
Article32. Bekir J, Mars M, Souchard JP, Bouajila J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (Punica granatum) leaves. Food Chem Toxicol. 2013; 55:470–475.
Article33. Kwak CS, Lee KJ, Chang JH, Park JH, Cho JH, Park JH, Kim KM, Lee MS. In vitro antioxidant and anti-inflammatory effects of ethanol extracts from Korean sweet potato leaves and stalks. J Korean Soc Food Sci Nutr. 2013; 42(3):369–377.34. Park SC, Lee MS, Kim HS. Investigate on aging-delay and aging-related disease prevention factors in Korean foods and construct database [Report to Ministry of Agriculture and Forestry]. Seoul: Seoul National University Medical Institute;2004.35. Lee MS, Jeong HK, Kwak CS. A study on the systemic recording of Korean healthy traditional foods in Honam district and its cultural commercialization [Report to Ministry of Agriculture, Food and Rural Affairs: 11-1543000-000118-01]. Daejeon: Hannam University;2013.36. Ham SA, Hwang JS, Kang ES, Yoo T, Lim HH, Lee WJ, Paek KS, Seo HG. Ethanol extract of Dalbergia odorifera protects skin keratinocytes against ultraviolet B-induced photoaging by suppressing production of reactive oxygen species. Biosci Biotechnol Biochem. 2015; 79(5):760–766.
Article37. Shindo Y, Witt E, Packer L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J Invest Dermatol. 1993; 100(3):260–265.
Article38. Shindo Y, Hashimoto T. Antioxidant defence mechanism of the skin against UV irradiation: study of the role of catalase using acatalasaemia fibroblasts. Arch Dermatol Res. 1995; 287(8):747–753.
Article39. Shindo Y, Witt E, Han D, Epstein W, Packer L. Enzymic and nonenzymic antioxidants in epidermis and dermis of human skin. J Invest Dermatol. 1994; 102(1):122–124.
Article40. Piao MJ, Kang KA, Kim KC, Chae S, Kim GO, Shin T, Kim HS, Hyun JW. Diphlorethohydroxycarmalol attenuated cell damage against UVB radiation via enhancing antioxidant effects and absorbing UVB ray in human HaCaT keratinocytes. Environ Toxicol Pharmacol. 2013; 36(2):680–688.
Article41. Liochev SI, Fridovich I. The role of O2.- in the production of HO.: in vitro and in vivo. Free Radic Biol Med. 1994; 16(1):29–33.
Article42. Sasaki H, Akamatsu H, Horio T. Protective role of copper, zinc superoxide dismutase against UVB-induced injury of the human keratinocyte cell line HaCaT. J Invest Dermatol. 2000; 114(3):502–507.
Article43. Hashimoto Y, Ohkuma N, Iizuka H. Reduced superoxide dismutase activity in UVB-induced hyperproliferative pig epidermis. Arch Dermatol Res. 1991; 283(5):317–320.
Article44. Song JL, Gao Y. Protective effects of Lindera coreana on UVB-induced oxidative stress in human HaCaT keratinocytes. Iran J Pharm Res. 2014; 13(4):1369–1378.45. Jeon SE, Choi-Kwon S, Park KA, Lee HJ, Park MS, Lee JH, Kwon SB, Park KC. Dietary supplementation of (+)-catechin protects against UVB-induced skin damage by modulating antioxidant enzyme activities. Photodermatol Photoimmunol Photomed. 2003; 19(5):235–241.
Article46. Panchenko MV, Farber HW, Korn JH. Induction of hemeoxygenase-1 by hypoxia and free radicals in human dermal fibroblasts. Am J Physiol Cell Physiol. 2000; 278(1):C92–C101.47. Obermüller-Jevic UC, Schlegel B, Flaccus A, Biesalski HK. The effect of β-carotene on the expression of interleukin-6 and heme oxygenase-1 in UV-irradiated human skin fibroblasts in vitro. FEBS Lett. 2001; 509(2):186–190.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Suppressive effects of ethanol extract of Aralia elata on UVB-induced oxidative stress in human keratinocytes
- Inhibitory effect of Aralia elata ethanol extract against skin damage in UVB-exposed human keratinocytes and human dermal fibroblasts
- Protective effect of an ethanol extract mixture of Aralia elata, Chaenomeles sinensis fruit, and Glycyrrhizae radix against cerebral ischemiareperfusion injury in rats and excitotoxic and oxidative neuronal damage
- Galangin (3,5,7-Trihydroxyflavone) Shields Human Keratinocytes from Ultraviolet B-Induced Oxidative Stress
- Inhibitory effect of an ethanol extract mixture of Aralia elata leaf, Chaenomeles sinensis fruit and Glycyrrhizae radix on Aβ (25–35)-induced memory impairment