Korean J Radiol.
2006 Dec;7(4):257-266. 10.3348/kjr.2006.7.4.257.
Transcatheter Arterial Chemoembolization of Hepatocellular Carcinoma: Prevalence and Causative Factors of Extrahepatic Collateral Arteries in 479 Patients
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Institute of Radiation Medicine, Seoul National University Medical Research Center, and Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. chungjw@radcom.s
- 2Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1092549
- DOI: http://doi.org/10.3348/kjr.2006.7.4.257
Abstract
OBJECTIVE
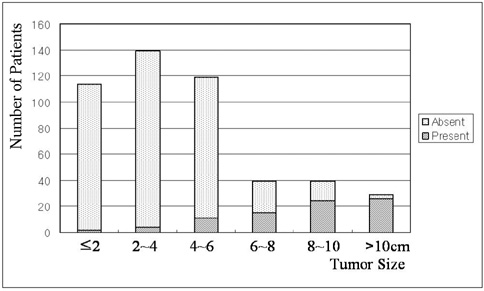
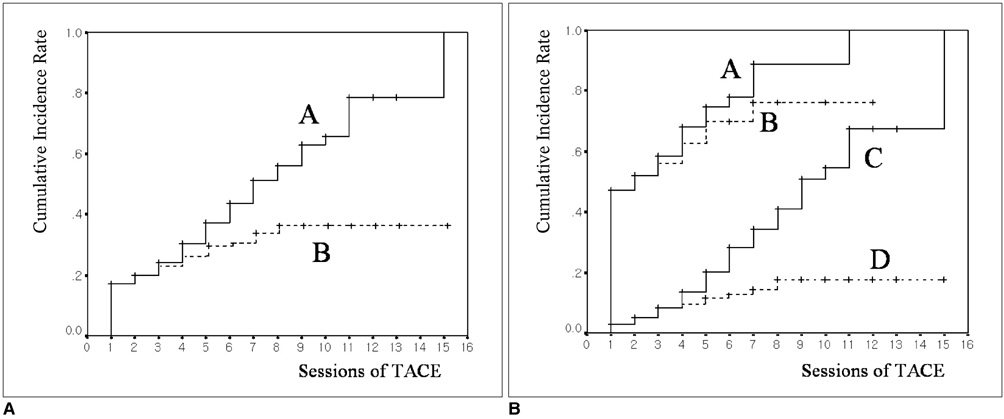
We wanted to investigate the prevalence and causative factors of extrahepatic arterial blood supply to hepatocellular carcinoma (HCC) at its initial presentation and during chemoembolization. MATERIALS AND METHODS: Between February 1998 and April 2000, consecutive 479 patients with newly diagnosed HCC were prospectively enrolled into this study. A total of 1629 sessions of transcatheter arterial chemoembolization (TACE) were performed in these patients (range: 1-15 sessions; mean: 3.4 sessions) until April 2004. For each TACE procedure, we determined the potential extrahepatic collateral arteries (ExCAs) depending on the location of the tumor, and we performed selective angiography of all suspected collaterals that could supply the tumor. The prevalence of ExCAs and the causative factors were analyzed. RESULTS: At initial presentation, 82 (17%) of these 479 patients showed 108 ExCAs supplying tumors. Univariate analysis showed that tumor size (p < 0.01), patient age (p = 0.02), a surface location (p < 0.01), and a bare area location (p < 0.01) were significantly associated with the presence of ExCAs. Multiple logistic regression analysis showed that only tumor size was predictive of ExCA formation (p < 0.01, odds ratio = 1.737, confidence interval: 1.533 to 1.969). During repeated TACE sessions, 97 additional ExCAs were detected in 70 (14%) patients. The cumulative probability of ExCAs in patients with a large tumor (> or = 5 cm) was significantly higher than that for those patients with a small tumor (< 5 cm) (p < 0.01). CONCLUSION: The presence of ExCAs supplying HCC is rather common, and the tumor size is a significant causative factor for the development of these collateral arteries.
Keyword
MeSH Terms
-
Neovascularization, Pathologic/*etiology/physiopathology/radiography
Middle Aged
Male
Logistic Models
Liver Neoplasms/physiopathology/*therapy
Humans
Female
Collateral Circulation/drug effects/physiology
Chemoembolization, Therapeutic/*methods
Carcinoma, Hepatocellular/physiopathology/*therapy
Angiography
Aged, 80 and over
Aged
Adult
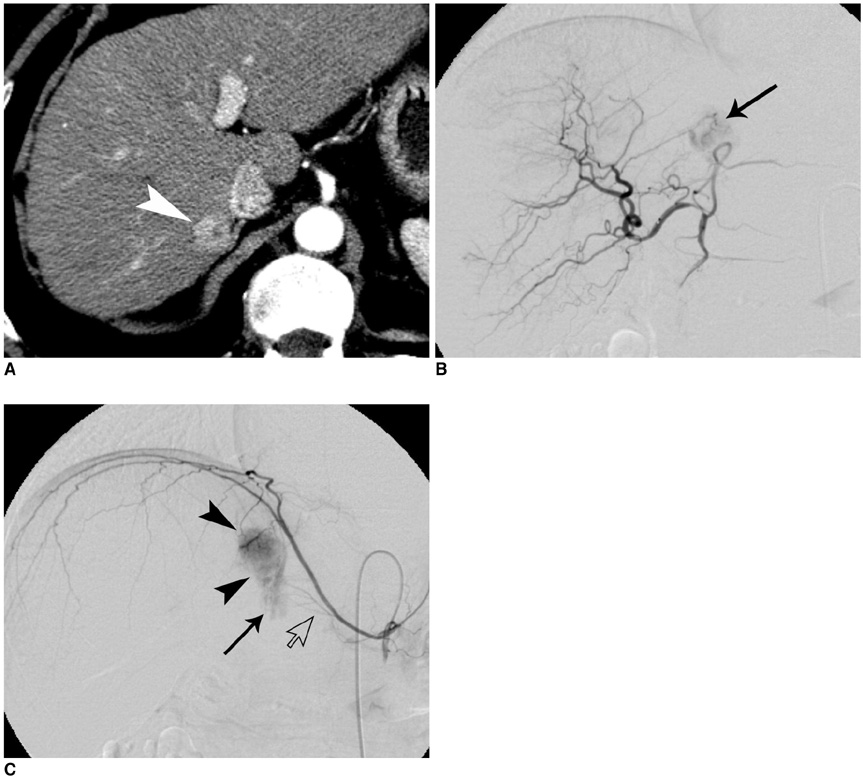
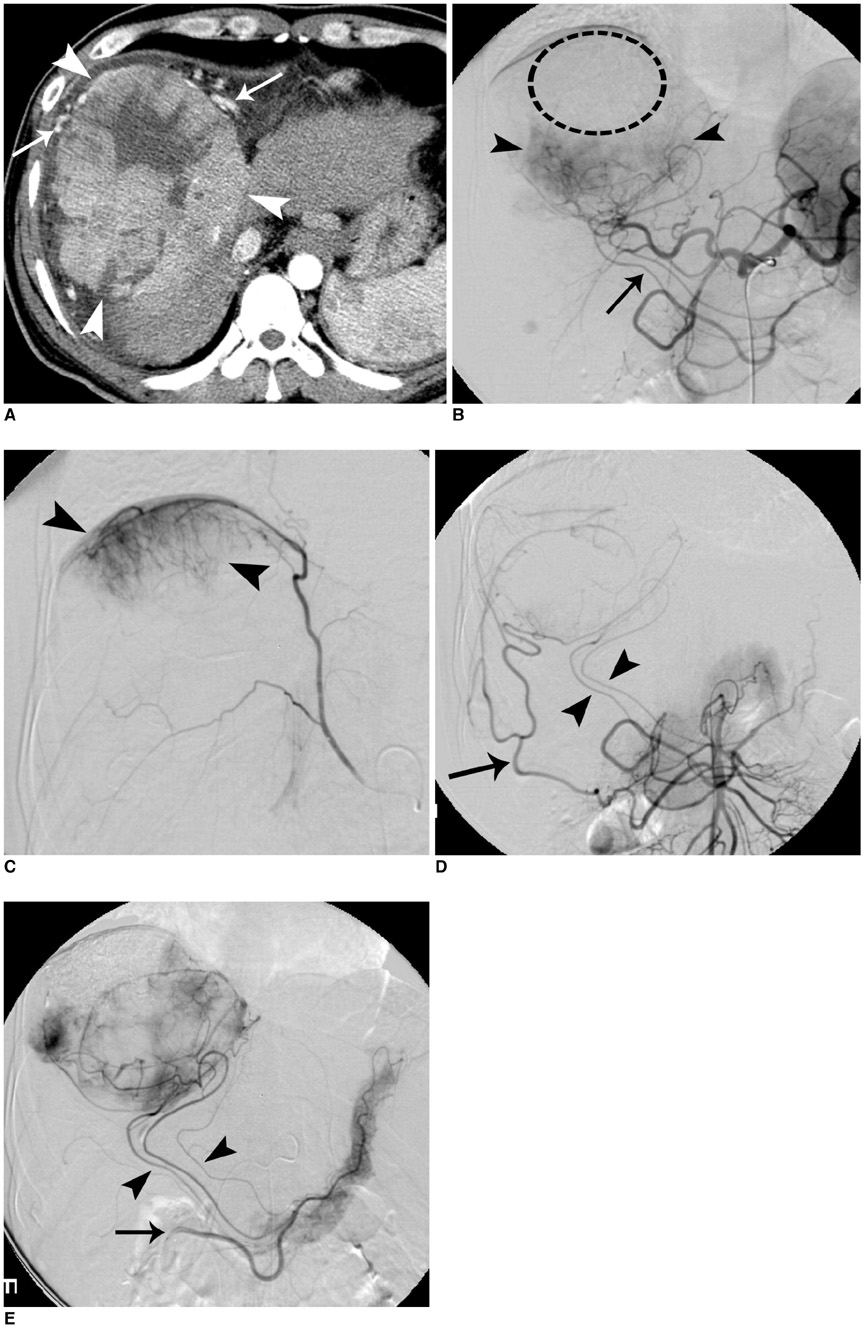
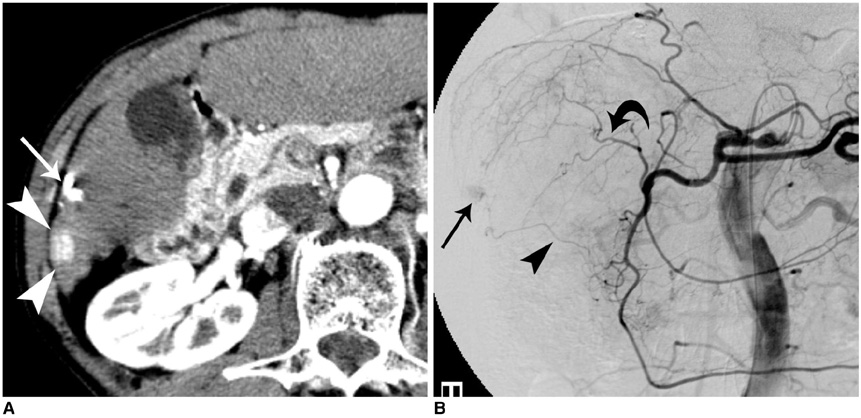
Figure
Reference
-
1. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999. 340:745–750.2. Carr BI. Hepatocellular carcinoma: current management and future trends. Gastroenterology. 2004. 127:S218–S224.3. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002. 35:1164–1171.4. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003. 37:429–442.5. Nakakuma K, Tashiro S, Hiraoka T, Uemura K, Konno T, Miyauchi Y, et al. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983. 52:2193–2200.6. Kim JH, Chung JW, Han JK, Park JH, Choi BI, Han MC. Transcatheter arterial embolization of the internal mammary artery in hepatocellular carcinoma. J Vasc Interv Radiol. 1995. 6:71–74.7. Chung JW, Park JH, Han JK, Choi BI, Kim TK, Han MC. Transcatheter oily chemoembolization of the inferior phrenic artery in hepatocellular carcinoma: the safety and potential therapeutic role. J Vasc Interv Radiol. 1998. 9:495–500.8. Kim HC, Chung JW, Lee W, Jae HJ, Park JH. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005. 25:S25–S39.9. Chung JW, Park JH, Han JK, Choi BI, Han MC, Lee HS, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996. 198:33–40.10. Kwon JW, Chung JW, Song SY, Lim HG, Myung JS, Choi YH, et al. Transcatheter arterial chemoembolization for hepatocellular carcinomas in patients with celiac axis occlusion. J Vasc Interv Radiol. 2002. 13:689–694.11. Song SY, Chung JW, Kwon JW, Joh JH, Shin SJ, Kim HB, et al. Collateral pathways in patients with celiac axis stenosis: angiographic-spiral CT correlation. Radiographics. 2002. 22:881–893.12. Nakai M, Sato M, Kawai N, Minamiguchi H, Masuda M, Tanihata H, et al. Hepatocellular carcinoma: involvement of the internal mammary artery. Radiology. 2001. 219:147–152.13. Miyayama S, Matsui O, Akakura Y, Yamamoto T, Nishida H, Yoneda K, et al. Hepatocellular carcinoma with blood supply from omental branches: treatment with transcatheter arterial embolization. J Vasc Interv Radiol. 2001. 12:1285–1290.14. Won JY, Lee DY, Lee JT, Park SI, Kim MJ, Yoo HS, et al. Supplemental transcatheter arterial chemoembolization through a collateral omental artery: treatment for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2003. 26:136–140.15. Park SI, Lee DY, Won JY, Lee JT. Extrahepatic collateral supply of hepatocellular carcinoma by the intercostal arteries. J Vasc Interv Radiol. 2003. 14:461–468.16. Miyayama S, Matsui O, Nishida H, Yamamori S, Minami T, Shinmura R, et al. Transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma fed by the cystic artery. J Vasc Interv Radiol. 2003. 14:1155–1161.17. Couinaud C. Le foie: études anatomiques et chirurgicales. 1957. Paris, France: Masson;9–12.18. Charnsangavej C, Chuang VP, Wallace S, Soo CS, Bowers T. Angiographic classification of hepatic arterial collaterals. Radiology. 1982. 144:485–494.19. Michels NA. Collateral arterial pathways to the liver after ligation of the hepatic artery and removal of the celiac axis. Cancer. 1953. 6:708–724.20. Doppman JL, Girton M, Kahn R. Proximal versus peripheral hepatic artery embolization experimental study in monkeys. Radiology. 1978. 128:577–588.21. Hirakawa M, Iida M, Aoyagi K, Matsui T, Akagi K, Fujishima M. Gastroduodenal lesions after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Am J Gastroenterol. 1988. 83:837–840.22. Arora R, Soulen MC, Haskal ZJ. Cutaneous complications of hepatic chemoembolization via extrahepatic collaterals. J Vasc Interv Radiol. 1999. 10:1351–1356.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An ischemic skin lesion after chemoembolization of the right internal mammary artery in a patient with hepatocellular carcinoma
- Palliative Transcatheter Arterial Chemoembolization for Relieving Metastatic Bone Pain due to Hepatocellular Carcinoma: A Case Report
- Rupture of hepatocellular carcinoma after transcatheter arterial chemoembolization: A case report
- A Fatal Case of Pulmonary Embolism after Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma
- Conventional Chemoembolization for Hepatocellular Carcinoma: Role of Cone-Beam Computed Tomography Guidance