J Vet Sci.
2010 Dec;11(4):285-289. 10.4142/jvs.2010.11.4.285.
Peripheral serotoninergic response to physical exercise in athletic horses
- Affiliations
-
- 1Department of Experimental Sciences and Applied Biotechnology, Faculty of Veterinary Medicine, University of Messina, Messina, Italy. dalberghina@unime.it
- KMID: 1072176
- DOI: http://doi.org/10.4142/jvs.2010.11.4.285
Abstract
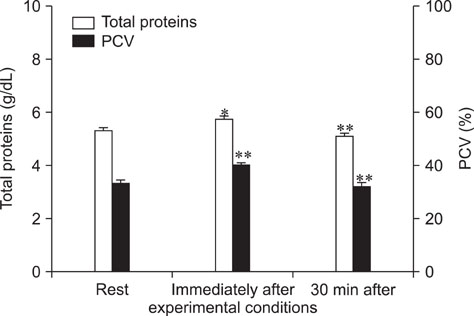
- The purpose of this study was to evaluate the influence of exercise on plasma tryptophan (TRP) and free serotonin (f5-HT), whole blood-5-HT (WB-5-HT) and f5-HT/WB-5-HT ratio in Italian Saddle horses. Six clinically healthy Italian Saddle horses were subjected to a 450 meters obstacles course. Blood samples were collected from each horse by jugular venipuncture using vacutainer tubes with K3-EDTA at rest, immediately after exercise, and after 30 min. TRP, f5-HT and WB-5-HT were analyzed by HPLC. Immediately after exercise, statistically significant increases of f5-HT (p<0.001) and WB-5-HT (p<0.001) were observed. After 30 min, f5-HT and WB-5-HT decreased compared to immediately after exercise, but were still significantly higher than rest values (p<0.01 and p<0.05, respectively). A significant linear regression between f5-HT and WB-5-HT was observed during experimental conditions. f5-HT and WB-5-HT modifications after exercise suggest an important role of peripheral serotoninergic markers in response to physical activity. The possible source of extra serotonin detected after show jumping should be clarified by further investigation.
Keyword
MeSH Terms
Figure
Reference
-
1. Alberghina D, Giannetto C, Visser EK, Ellis AD. Effect of diet on plasma tryptophan and serotonin in trained mares and geldings. Vet Rec. 2010. 166:133–136.
Article2. Alberghina D, Amorini AM, Lazzarino G. Modulation of peripheral markers of the serotoninergic system in healthy horses. Res Vet Sci. 2010. Epub ahead of print. doi: 10.1016/j.rvsc.2010.06.023.
Article3. Anderson GM, Feibel FC, Cohen DJ. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life Sci. 1987. 40:1063–1070.
Article4. Arida RM, Naffah-Mazzacoratti Mda G, Soares J, Cavalheiro EA. Monoamine responses to acute and chronic aerobic exercise in normotensive and hypertensive subjects. Sao Paulo Med J. 1998. 116:1618–1624.
Article5. Bailey SP, Davis JM, Ahlborn EN. Serotonergic agonists and antagonists affect endurance performance in the rat. Int J Sports Med. 1993. 14:330–333.
Article6. Baptista S, Piloto N, Reis F, Teixeira-de-Lemos E, Garrido AP, Dias A, Lourenço M, Palmeiro A, Ferrer-Antunes C, Teixeira F. Treadmill running and swimming imposes distinct Cardiovascular physiological adaptations in the rat: focus on serotonergic and sympathetic nervous systems modulation. Acta Physiol Hung. 2008. 95:365–381.
Article7. Bergero D, Assenza A, Schiavone A, Piccione G, Perona G, Caola G. Amino acid concentrations in blood serum of horses performing long lasting low-intensity exercise. J Anim Physiol Anim Nutr (Berl). 2005. 89:146–150.
Article8. Bianchi M, Moser C, Lazzarini C, Vecchiato E, Crespi F. Forced swimming test and fluoxetine treatment: in vivo evidence that peripheral 5-HT in rat platelet-rich plasma mirrors cerebral extracellular 5-HT levels, whilst 5-HT in isolated platelets mirrors neuronal 5-HT changes. Exp Brain Res. 2002. 143:191–197.
Article9. Blomstrand E, Celsing F, Newsholme EA. Changes in plasma concentrations of aromatic and branched-chain amino acids during sustained exercise in man and their possible role in fatigue. Acta Physiol Scand. 1988. 133:115–121.
Article10. Bülbring E, Crema A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol Chemother. 1958. 13:444–457.
Article11. Bülbring E, Lin RCY. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to Intraluminal pressure and propulsive activity. J Physiol. 1958. 140:381–407.12. Chaouloff F, Laude D, Elghozi JL. Physical exercise: evidence for differential Consequences of tryptophan on 5-HT synthesis and metabolism in central serotonergic cell bodies and terminals. J Neural Transm. 1989. 78:121–130.
Article13. Côté F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med. 2004. 10:232–238.
Article14. Da Prada M, Cesura AM, Launay JM, Richards JG. Platelets as a model for neurones? Experientia. 1988. 44:115–126.
Article15. Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc. 1997. 29:45–57.
Article16. Delesalle C, Deprez P, Schuurkes JA, Lefebvre RA. Contractile effects of 5-hydroxytryptamine and 5-carboxamidotryptamine in the equine jejunum. Br J Pharmacol. 2006. 147:23–35.
Article17. Farris JW, Hinchcliff KW, McKeever KH, Lamb DR, Thompson DL. Effect of tryptophan and of glucose on exercise capacity of horses. J Appl Physiol. 1998. 85:807–816.18. Fernstrom JD, Fernstrom MH. Exercise, serum free tryptophan, and central fatigue. J Nutr. 2006. 136:553S–559S.
Article19. Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience. 1982. 7:341–349.
Article20. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001. 81:1725–1789.
Article21. Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998. 115:370–380.
Article22. Hackl S, van den Hoven R, Zickl M, Spona J, Zentek J. The effects of short intensive exercise on plasma free amino acids in standardbred trotters. J Anim Physiol Anim Nutr (Berl). 2009. 93:165–173.
Article23. Hara K, Hirowatari Y, Yoshika M, Komiyama Y, Tsuka Y, Takahashi H. The ratio of plasma to whole-blood serotonin may be a novel marker of atherosclerotic cardiovascular disease. J Lab Clin Med. 2004. 144:31–37.
Article24. Haritou SJA, Zylstra R, Ralli C, Turner S, Tortonese DJ. Seasonal changes in circadian peripheral plasma concentrations of melatonin, serotonin, dopamine and cortisol in aged horses with Cushing's disease under natural photoperiod. J Neuroendocrinol. 2008. 20:988–996.
Article25. Kremer HP, Goekoop JG, Van Kempen GM. Clinical use of the determination of serotonin in whole blood. J Clin Psychopharmacol. 1990. 10:83–87.
Article26. Maurer-Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost. 2004. 91:119–128.
Article27. McMenamy RH, Oncley JL. The specific binding of L-tryptophan to serum albumin. J Biol Chem. 1958. 233:1436–1447.
Article28. McPhee SJ, Ganong WF. Pathophysiology of Disease: An Introduction to Clinical Medicine. 2006. New York: McGraw Hill;784.29. Nakatani Y, Sato-Suzuki I, Tsujino N, Nakasato A, Seki Y, Fumoto M, Arita H. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur J Neurosci. 2008. 27:2466–2472.
Article30. Piccione G, Assenza A, Fazio F, Percipalle M, Caola G. Central fatigue and nycthemeral change of serum tryptophan and serotonin in the athletic horse. J Circadian Rhythms. 2005. 3:6.
Article31. Sharma HS, Westman J, Navarro JC, Dey PK, Nyberg F. Probable involvement of serotonin in the increased permeability of the blood-brain barrier by forced swimming. An experimental study using Evans blue and 131I-sodium tracers in the rat. Behav Brain Res. 1995. 72:189–196.
Article32. Struder HK, Hollmann W, Platen P, Duperly J, Fischer HG, Weber K. Alterations in plasma free tryptophan and large neutral amino acids do not affect perceived exertion and prolactin during 90 min of treadmill exercise. Int J Sports Med. 1996. 17:73–79.
Article33. Tyce GM. Origin and metabolism of serotonin. J Cardiovasc Pharmacol. 1990. 16:Suppl. S1–S7.
Article34. van Hall G, Raaymakers JS, Saris WH, Wagenmakers AJ. Ingestion of branched-chain amino acids and tryptophan during sustained exercise in man: failure to affect performance. J Physiol. 1995. 486:789–794.
Article35. Vervuert I, Coenen M, Watermülder E. Metabolic responses to oral tryptophan supplementation before exercise in horses. J Anim Physiol Anim Nutr (Berl). 2005. 89:140–145.
Article36. Weiss R, Abel D, Scholtysik G, Straub R, Mevissen M. 5-Hydroxytryptamine mediated contractions in isolated preparations of equine ileum and pelvic flexure: pharmacological characterization of a new 5-HT(4) agonist. J Vet Pharmacol Ther. 2002. 25:49–58.
Article37. Yuwiler A, Oldendorf WH, Geller E, Braun L. Effect of albumin binding and amino acid competition on tryptophan uptake into brain. J Neurochem. 1977. 28:1015–1023.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasound and clinical findings in the metacarpophalangeal joint assessment of show jumping horses in training
- Integrated analysis of microRNA and mRNA expressions in peripheral blood leukocytes of Warmblood horses before and after exercise
- A novel biomarker of exercise-induced stress in horses
- Clinical assessment and grading of back pain in horses
- Effect of Short Termed Fasting on the Usage Patterns of Metabolic Energy Sources during Exercise in Man