J Korean Acad Conserv Dent.
2004 May;29(3):226-232. 10.5395/JKACD.2004.29.3.226.
Measurement of the excessive stimulus time after the sensory threshold level during electric pulp testing
- Affiliations
-
- 1Department of Medical Engineering, College of Medicine, Yonsei University, Korea.
- 2Department of Conservative Dentistry, College of Dentistry, Yonsei University, Korea. sjlee@yumc.yonsei.ac.kr
- KMID: 1987098
- DOI: http://doi.org/10.5395/JKACD.2004.29.3.226
Abstract
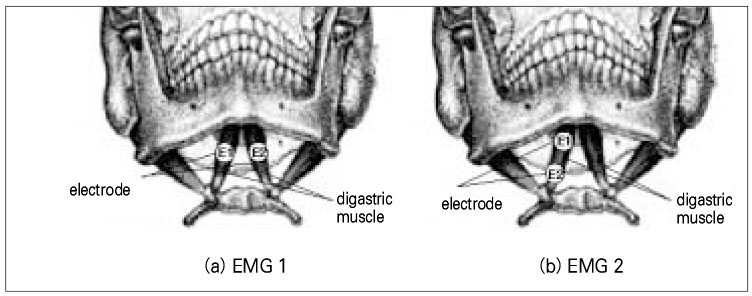
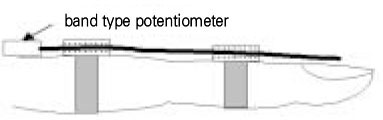
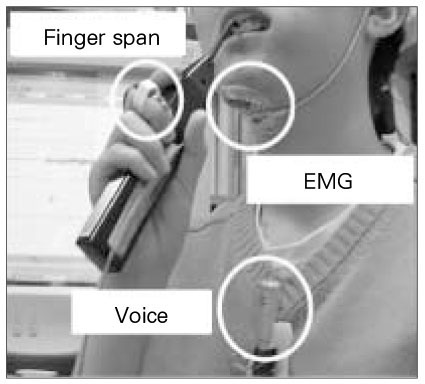
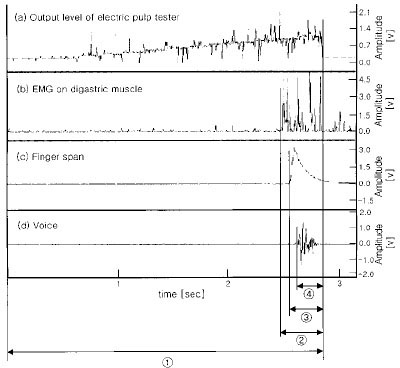
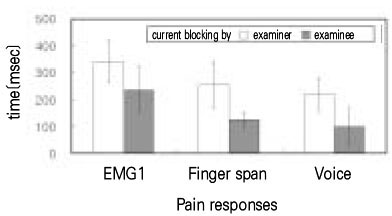
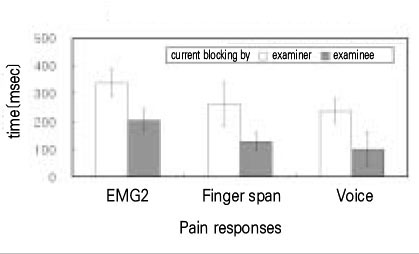
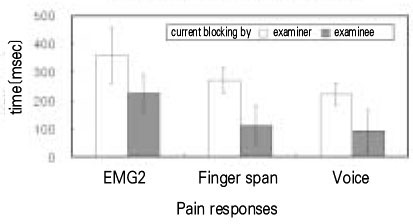
- Use of electric pulp testing elicits painful response in vital teeth. In this study, we examined the excessive time from pain feeling to stimulation disconnection in clinical situation. D626D (Parkell Inc., USA.) scan type electric pulp tester was used in total of 23 young healthy individuals. Each of the right central incisors and first premolars were used as testing teeth. Stimulation disconnection was achieved by EMG in anterior belly of digastric muscle, finger span, and voice and the excessive stimulation time over the sensory threshold was recorded. As a result, we found that the short responses before the stimulation disconnection appeared following order; EMG, finger span, and voice. The EMG disconnection is suggested to be used to reduce the excessive stimulus time in electric pulp testing.
Keyword
Figure
Cited by 1 articles
-
Evaluation of the change of lower lip sensation after inferior alveolar nerve block by using the electric pulp tester
Myong-Suk Ku, Jin Wook Kim, Young Hoon Jeon, Tae Geon Kwon, Sang Han Lee
J Korean Assoc Oral Maxillofac Surg. 2011;37(6):464-469. doi: 10.5125/jkaoms.2011.37.6.464.
Reference
-
1. Ingle JI, Taintor JF. Endontics. 1965. 3rd edition. Lea & Febiger.2. Byers MR. Dental sonsory receptor. Int Rev Neurobiol. 1984. 25:39–94.3. Närhi MV. The characteristics of intradental sensory units and their responses to stimulation. J Dent Res. 1985. 64 Spec No:564–571.
Article4. Brannstrom M, Johnson G, Nordenvall KJ. Transmission and control of dental pain: resin impregnation for the desensitization of dentin. J Am Dent Assoc. 1979. 99(4):612–618.
Article5. Hirvonen TJ, Narhi MVO, Hakumaku MOK. The excitability of dog pulp nerves in relation to the condition of dentine surface. J Endod. 1984. 10(7):294–298.
Article6. Narhi MVO, Hirvonen TJ, Hakumaki MOK. Activation of intradental nerves in the dog to some stimuli applied to the dentine. Arch Oral Biol. 1982. 27(12):1053–1058.
Article7. Narhi MVO, Hirvonen TJ, Hakumaki MOK. Responses of intradental nerve fibres to stimulation of dentine and pulp. Acta Physiol Scand. 1982. 115(2):173–178.
Article8. Azerad J, Woda A, Fessard DA. Physiological properties of neurons in different parts of cat trigeminal sensory complex. Brain Res. 1982. 246(1):7–21.
Article9. Mahan PE. Jaw depression elicited by tooth pulp stimulation. Exp Neurol. 1970. 29(3):439–448.
Article10. Mason P, Strassman A, Maciewicz R. Is the Jaw-Opening Refelx a valid model of pain? Brain Res. 1985. 357:137–146.11. Koole P, Jongh HJD, Boering G. A comparative study of electromyograms of the masseter, temporalis, and anterior digastric muscles obtained by surface and intramuscular electrodes: raw-EMG. Cranio. 1991. 9(3):228–240.
Article12. Matthews B, Baxter J, Watts S. Sensory and reflex responses to tooth pulp stimulation in man. Brain Res. 1976. 113(1):83–94.
Article13. Han YC, Lee JS, Lee JH. Extracellular recording in trigeminal subnucleus caudalis of the anesthetized cat produced by electrical pulp stimulation. Korean J Oral Biol. 1985. 9(1):21–29.14. Yu MG, Park SJ. Changes in autonomic responses and jaw muscle activity induced by tooth pulp stimulation in the rat. J Korean Acad Conserv Dent. 1999. 24(1):657–665.15. Ahlquist ML, Edwall LGA, Franzen OG, Haegerstam GAT. Perception of pulpal pain as a fuction of intradental nerve activity. Pain. 1984. 19(4):353–366.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement of the excessive stimulus time after the sensory threshold level during electric pulp testing
- The Preliminary Study for Nomative Current perception Threshold values in the Korean Adults
- Evaluation of the change of lower lip sensation after inferior alveolar nerve block by using the electric pulp tester
- Significant parameters of the bulbocavernosus reflex latency testing for the diagnosis of neurogenic impotence
- Comparison of Olfactory Threshold Tests:CCCRC Test versus Step Method