Allergy Asthma Immunol Res.
2014 Sep;6(5):389-400. 10.4168/aair.2014.6.5.389.
Environmental Changes, Microbiota, and Allergic Diseases
- Affiliations
-
- 1Department of Pediatrics, Inje University Haeundae Paik Hospital, Busan, Korea.
- 2Department of Pediatrics, Hallym University Sacred Heart Hospital, University of Hallym College of Medicine, Anyang, Korea.
- 3Department of Pediatrics, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 4Department of Pediatrics, Childhood Asthma Atopy Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 5Research Center for Standardization of Allergic Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2181074
- DOI: http://doi.org/10.4168/aair.2014.6.5.389
Abstract
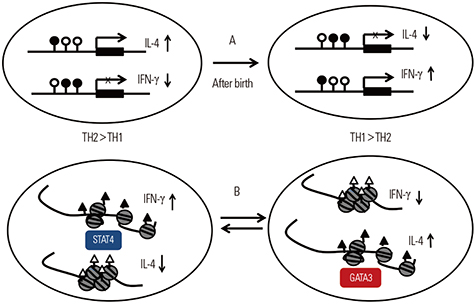
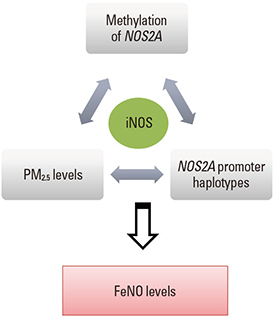
- During the last few decades, the prevalence of allergic disease has increased dramatically. The development of allergic diseases has been attributed to complex interactions between environmental factors and genetic factors. Of the many possible environmental factors, most research has focused on the most commonly encountered environmental factors, such as air pollution and environmental microbiota in combination with climate change. There is increasing evidence that such environmental factors play a critical role in the regulation of the immune response that is associated with allergic diseases, especially in genetically susceptible individuals. This review deals with not only these environmental factors and genetic factors but also their interactions in the development of allergic diseases. It will also emphasize the need for early interventions that can prevent the development of allergic diseases in susceptible populations and how these interventions can be identified.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Gene-Environment Interactions in Asthma: Genetic and Epigenetic Effects
Jong-Uk Lee, Jeong Dong Kim, Choon-Sik Park
Yonsei Med J. 2015;56(4):877-886. doi: 10.3349/ymj.2015.56.4.877.Can the Use of Antibiotics Alter the Susceptibility to Allergic Diseases?
So-Yeon Lee
Allergy Asthma Immunol Res. 2018;10(5):425-427. doi: 10.4168/aair.2018.10.5.425.Microbiome in the Gut-Skin Axis in Atopic Dermatitis
So-Yeon Lee, Eun Lee, Yoon Mee Park, Soo-Jong Hong
Allergy Asthma Immunol Res. 2018;10(4):354-362. doi: 10.4168/aair.2018.10.4.354.
Reference
-
1. Balbus JM, Barouki R, Birnbaum LS, Etzel RA, Gluckman PD Sr, Grandjean P, Hancock C, Hanson MA, Heindel JJ, Hoffman K, Jensen GK, Keeling A, Neira M, Rabadan-Diehl C, Ralston J, Tang KC. Early-life prevention of non-communicable diseases. Lancet. 2013; 381:3–4.2. Renz H, von Mutius E, Brandtzaeg P, Cookson WO, Autenrieth IB, Haller D. Gene-environment interactions in chronic inflammatory disease. Nat Immunol. 2011; 12:273–277.3. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115:1111–1119.4. Greer RL, Morgun A, Shulzhenko N. Bridging immunity and lipid metabolism by gut microbiota. J Allergy Clin Immunol. 2013; 132:253–262.5. Prescott SL, Clifton V. Asthma and pregnancy: emerging evidence of epigenetic interactions in utero. Curr Opin Allergy Clin Immunol. 2009; 9:417–426.6. Barne C, Alexis NE, Bernstein JA, Cohn JR, Demain JG, Horner E, Levetin E, Nel A, Phipatanakul W. Climate Change and Our Environment: The Effect on Respiratory and Allergic Disease. J Allergy Clin Immunol Pract. 2013; 1:137–141.7. Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009; 27:391–402.8. Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. 2013; 131:23–30.9. Bielory L, Lyons K, Goldberg R. Climate change and allergic disease. Curr Allergy Asthma Rep. 2012; 12:485–494.10. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867.11. D'Vaz N, Meldrum SJ, Dunstan JA, Martino D, McCarthy S, Metcalfe J, Tulic MK, Mori TA, Prescott SL. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics. 2012; 130:674–682.12. Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011; 127:724–733.e1-30.13. Allan K, Kelly FJ, Devereux G. Antioxidants and allergic disease: a case of too little or too much? Clin Exp Allergy. 2010; 40:370–380.14. D'Vaz N, Meldrum SJ, Dunstan JA, Lee-Pullen TF, Metcalfe J, Holt BJ, Serralha M, Tulic MK, Mori TA, Prescott SL. Fish oil supplementation in early infancy modulates developing infant immune responses. Clin Exp Allergy. 2012; 42:1206–1216.15. Barden AE, Mori TA, Dunstan JA, Taylor AL, Thornton CA, Croft KD, Beilin LJ, Prescott SL. Fish oil supplementation in pregnancy lowers F2-isoprostanes in neonates at high risk of atopy. Free Radic Res. 2004; 38:233–239.16. Kim BJ, Kwon JW, Seo JH, Kim HB, Lee SY, Park KS, Yu J, Kim HC, Leem JH, Sakong J, Kim SY, Lee CG, Kang DM, Ha M, Hong YC, Kwon HJ, Hong SJ. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann Allergy Asthma Immunol. 2011; 107:214–219.e1.17. West CE, Videky DJ, Prescott SL. Role of diet in the development of immune tolerance in the context of allergic disease. Curr Opin Pediatr. 2010; 22:635–641.18. Lee SY, Yu J, Ahn KM, Kim KW, Shin YH, Lee KS, Hong SA, Jung YH, Lee E, Yang SI, Seo JH, Kwon JW, Kim BJ, Kim HB, Kim WK, Song DJ, Jang GC, Shim JY, Lee SY, Kwon JY, Choi SJ, Lee KJ, Park HJ, Won HS, Yoo HS, Kang MJ, Kim HY, Hong SJ. Additive effect between IL-13 polymorphism and cesarean section delivery/prenatal antibiotics use on atopic dermatitis: a birth cohort study (COCOA). PLoS One. 2014; 9(5):e96603.19. Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011; 12:9–23.20. Yu J, Jang SO, Kim BJ, Song YH, Kwon JW, Kang MJ, Choi WA, Jung HD, Hong SJ. The Effects of Lactobacillus rhamnosus on the Prevention of Asthma in a Murine Model. Allergy Asthma Immunol Res. 2010; 2:199–205.21. Marsland BJ, Yadava K, Nicod LP. The airway microbiome and disease. Chest. 2013; 144:632–637.22. Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013; 11:639–647.23. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Climate Change 2007: The Physical Science Basis. NY, USA: Cambridge University Press;2007. cited 2014 Jan 20. Available from: http://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_wg1_report_the_physical_science_basis.htm.24. Emberlin J, Detandt M, Gehrig R, Jaeger S, Nolard N, Rantio-Lehtimaki A. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int J Biometeorol. 2002; 46:159–170.25. Rogers CA, Wayne PM, Macklin EA, Muilenberg ML, Wagner CJ, Epstein PR, Bazzaz FA. Interaction of the onset of spring and elevated atmospheric CO2 on ragweed (Ambrosia artemisiifolia L.) pollen production. Environ Health Perspect. 2006; 114:865–869.26. D'Amato G, Liccardi G, Frenguelli G. Thunderstorm-asthma and pollen allergy. Allergy. 2007; 62:11–16.27. Freye HB, King J, Litwin CM. Variations of pollen and mold concentrations in 1998 during the strong El Nino event of 1997-1998 and their impact on clinical exacerbations of allergic rhinitis, asthma, and sinusitis. Allergy Asthma Proc. 2001; 22:239–247.28. Stafoggia M, Schwartz J, Forastiere F, Perucci CA. SISTI Group. Does temperature modify the association between air pollution and mortality? A multicity case-crossover analysis in Italy. Am J Epidemiol. 2008; 167:1476–1485.29. Strachan DP. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000; 55:Suppl 1. S2–S10.30. Lee SY, Kwon JW, Seo JH, Song YH, Kim BJ, Yu J, Park KS, Kim H, Kim EJ, Lee JS, Hong SJ. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol. 2012; 158:168–174.31. Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009; 7:887–894.32. Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997; 159:1739–1745.33. Kim JH, Ohsawa M. Oral tolerance to ovalbumin in mice as a model for detecting modulators of the immunologic tolerance to a specific antigen. Biol Pharm Bull. 1995; 18:854–858.34. Rask C, Evertsson S, Telemo E, Wold AE. A full flora, but not monocolonization by Escherichia coli or lactobacilli, supports tolerogenic processing of a fed antigen. Scand J Immunol. 2005; 61:529–535.35. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012; 486:222–227.36. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010; 107:11971–11975.37. Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008; 38:634–642.38. Adlerberth I, Strachan DP, Matricardi PM, Ahrne S, Orfei L, Aberg N, Perkin MR, Tripodi S, Hesselmar B, Saalman R, Coates AR, Bonanno CL, Panetta V, Wold AE. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007; 120:343–350.39. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, Stokholm J, Smith B, Krogfelt KA. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011; 128:646–652.e1-5.40. Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012; 129:434–440.e1-2.41. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002; 196:1645–1651.42. Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, Karisola P, Auvinen P, Paulin L, Makela MJ, Vartiainen E, Kosunen TU, Alenius H, Haahtela T. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci U S A. 2012; 109:8334–8339.43. Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N, Gordon JI, Knight R. Moving pictures of the human microbiome. Genome Biol. 2011; 12:R50.44. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008; 455:1109–1113.45. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444:1027–1031.46. von Hertzen LC, Joensuu H, Haahtela T. Microbial deprivation, inflammation and cancer. Cancer Metastasis Rev. 2011; 30:211–223.47. Schaub B, Liu J, Hoppler S, Schleich I, Huehn J, Olek S, Wieczorek G, Illi S, von Mutius E. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009; 123:774–782.e5.48. McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002; 359:386–391.49. Kim BJ, Lee SY, Kwon JW, Jung YH, Lee E, Yang SI, Kim HY, Seo JH, Kim HB, Kim HC, Leem JH, Kwon HJ, Hong SJ. Traffic-related air pollution is associated with airway hyperresponsiveness. J Allergy Clin Immunol. 2014; Forthcoming.50. Kim BJ, Seo JH, Jung YH, Kim HY, Kwon JW, Kim HB, Lee SY, Park KS, Yu J, Kim HC, Leem JH, Lee JY, Sakong J, Kim SY, Lee CG, Kang DM, Ha M, Hong YC, Kwon HJ, Hong SJ. Air pollution interacts with past episodes of bronchiolitis in the development of asthma. Allergy. 2013; 68:517–523.51. Jeong SH, Kim JH, Son BK, Hong SC, Kim SY, Lee GH, Lim DH. Comparison of air pollution and the prevalence of allergy-related diseases in Incheon and Jeju City. Korean J Pediatr. 2011; 54:501–506.52. Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J. GINI Study Group. LISA Study Group. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008; 177:1331–1337.53. Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, Ahn K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013; 132:495–498.e1.54. Bråbäck L, Forsberg B. Does traffic exhaust contribute to the development of asthma and allergic sensitization in children: findings from recent cohort studies. Environ Health. 2009; 8:17.55. Alberg T, Nilsen A, Hansen JS, Nygaard UC, Lovik M. Nitrogen dioxide: no influence on allergic sensitization in an intranasal mouse model with ovalbumin and diesel exhaust particles. Inhal Toxicol. 2011; 23:268–276.56. Pawankar R, editor. World allergy organization (WAO) white book on allergy. WAO;2011.57. WHO Guidelines for Indoor Air Quality. Dampness and Mould. Geneva: World Health Organization;2009.58. Wallace LA, Mitchell H, O'Connor GT, Neas L, Lippmann M, Kattan M, Koenig J, Stout JW, Vaughn BJ, Wallace D, Walter M, Adams K, Liu LJ. Inner-City Asthma Study. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003; 111:1265–1272.59. Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, Gauderman WJ, Peters JM. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2002; 166:457–463.60. Yi O, Kwon HJ, Kim H, Ha M, Hong SJ, Hong YC, Leem JH, Sakong J, Lee CG, Kim SY, Kang D. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res. 2012; 113:40–45.61. Rabinovitch N, Silveira L, Gelfand EW, Strand M. The response of children with asthma to ambient particulate is modified by tobacco smoke exposure. Am J Respir Crit Care Med. 2011; 184:1350–1357.62. Perzanowski MS, Chew GL, Divjan A, Jung KH, Ridder R, Tang D, Diaz D, Goldstein IF, Kinney PL, Rundle AG, Camann DE, Perera FP, Miller RL. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013; 131:886–893.63. Herberth G, Gubelt R, Roder S, Kramer U, Schins RP, Diez U, Borte M, Heinrich J, Wichmann HE, Herbarth O, Lehmann I. LISAplus study group. Increase of inflammatory markers after indoor renovation activities: the LISA birth cohort study. Pediatr Allergy Immunol. 2009; 20:563–570.64. Choi H, Schmidbauer N, Sundell J, Hasselgren M, Spengler J, Bornehag CG. Common household chemicals and the allergy risks in pre-school age children. PLoS One. 2010; 5:e13423.65. Galaris D, Evangelou A. The role of oxidative stress in mechanisms of metal-induced carcinogenesis. Crit Rev Oncol Hematol. 2002; 42:93–103.66. Weichenthal S, Moase C, Chan P. A review of pesticide exposure and cancer incidence in the Agricultural Health Study cohort. Environ Health Perspect. 2010; 118:1117–1125.67. Pereira MA, Kramer PM, Conran PB, Tao L. Effect of chloroform on dichloroacetic acid and trichloroacetic acid-induced hypomethylation and expression of the c-myc gene and on their promotion of liver and kidney tumors in mice. Carcinogenesis. 2001; 22:1511–1519.68. Saxon A, Diaz-Sanchez D. Air pollution and allergy: you are what you breathe. Nat Immunol. 2005; 6:223–226.69. Salam MT, Byun HM, Lurmann F, Breton CV, Wang X, Eckel SP, Gilliland FD. Genetic and epigenetic variations in inducible nitric oxide synthase promoter, particulate pollution, and exhaled nitric oxide levels in children. J Allergy Clin Immunol. 2012; 129:232–239.e1-7.70. Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bollati V, Marinelli B, Cantone L, Rizzo G, Hou L, Schwartz J, Bertazzi PA, Baccarelli A. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation. Environ Health Perspect. 2009; 117:217–222.71. Liu J, Ballaney M, Al-alem U, Quan C, Jin X, Perera F, Chen LC, Miller RL. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci. 2008; 102:76–81.72. Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009; 180:462–467.73. Kohli A, Garcia MA, Miller RL, Maher C, Humblet O, Hammond SK, Nadeau K. Secondhand smoke in combination with ambient air pollution exposure is associated with increasedx CpG methylation and decreased expression of IFN-gamma in T effector cells and Foxp3 in T regulatory cells in children. Clin Epigenetics. 2012; 4:17.74. Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol. 2009; 40:464–473.75. Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005; 127:1232–1241.76. Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA methylation of 5'-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009; 4:e4488.77. Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J, Schneider-Stock R, Waterland RA, Bauer UM, von Mutius E, Garn H, Pfefferle PI, Renz H. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol. 2011; 128:618–625.e1-7.78. Vuillermin PJ, Ponsonby AL, Saffery R, Tang ML, Ellis JA, Sly P, Holt P. Microbial exposure, interferon gamma gene demethylation in naive T-cells, and the risk of allergic disease. Allergy. 2009; 64:348–353.79. Ziyab AH, Karmaus W, Holloway JW, Zhang H, Ewart S, Arshad SH. DNA methylation of the filaggrin gene adds to the risk of eczema associated with loss-of-function variants. J Eur Acad Dermatol Venereol. 2013; 27:e420–e423.80. Kauffmann F, Demenais F. Gene-environment interactions in asthma and allergic diseases: challenges and perspectives. J Allergy Clin Immunol. 2012; 130:1229–1240. quiz 1241-2.81. Thomas DC. Genetic epidemiology with a capital E: where will we be in another 10 years? Genet Epidemiol. 2012; 36:179–182.82. Ege MJ, Strachan DP, Cookson WO, Moffatt MF, Gut I, Lathrop M, Kabesch M, Genuneit J, Buchele G, Sozanska B, Boznanski A, Cullinan P, Horak E, Bieli C, Braun-Fahrlander C, Heederik D, von Mutius E; GABRIELA Study Group. Gene-environment interaction for childhood asthma and exposure to farming in Central Europe. J Allergy Clin Immunol. 2011; 127:138–144.e1-4.83. Lee SY, Kang MJ, Kwon JW, Park KS, Hong SJ. Breastfeeding Might Have Protective Effects on Atopy in Children With the CD14C-159T CT/CC Genotype. Allergy Asthma Immunol Res. 2013; 5:239–241.84. Kang SH, Jung YH, Kim HY, Seo JH, Lee JY, Kwon JW, Kim BJ, Kim HB, Lee SY, Jang GC, Song DJ, Kim WK, Shim JY, Kim JH, Kang MJ, Yu HS, Yu J, Hong SJ. Effect of paracetamol use on the modification of the development of asthma by reactive oxygen species genes. Ann Allergy Asthma Immunol. 2013; 110:364–369.e1.85. Tischer CG, Gref A, Standl M, Bauer M, Bergstrom A, Brauer M, Carlsten C, Gehring U, Granell R, Henderson J, Kerkhof M, MacNutt M, Melen E, Wickman M, Heinrich J. Glutathione-S-transferase P1, early exposure to mould in relation to respiratory and allergic health outcomes in children from six birth cohorts. A meta-analysis. Allergy. 2013; 68:339–346.86. Moreno-Macías H, Dockery DW, Schwartz J, Gold DR, Laird NM, Sienra-Monge JJ, Del Rio-Navarro BE, Ramirez-Aguilar M, Barraza-Villarreal A, Li H, London SJ, Romieu I. Ozone exposure, vitamin C intake, and genetic susceptibility of asthmatic children in Mexico City: a cohort study. Respir Res. 2013; 14:14.87. Fuertes E, Brauer M, MacIntyre E, Bauer M, Bellander T, von Berg A, Berdel D, Brunekreef B, Chan-Yeung M, Gehring U, Herbarth O, Hoffmann B, Kerkhof M, Klumper C, Koletzko S, Kozyrskyj A, Kull I, Heinrich J, Melen E, Pershagen G, Postma D, Tiesler CM, Carlsten C. TAG Study Group. Childhood allergic rhinitis, traffic-related air pollution, and variability in the GSTP1, TNF, TLR2, and TLR4 genes: results from the TAG Study. J Allergy Clin Immunol. 2013; 132:342–352.e2.88. Lee JY, Seo JH, Kwon JW, Yu J, Kim BJ, Lee SY, Kim HB, Kim WK, Kim KW, Shin YJ, Hong SJ. Exposure to gene-environment interactions before 1 year of age may favor the development of atopic dermatitis. Int Arch Allergy Immunol. 2012; 157:363–371.89. Kim WK, Kwon JW, Seo JH, Kim HY, Yu J, Kim BJ, Kim HB, Lee SY, Kim KW, Kang MJ, Shin YJ, Hong SJ. Interaction between IL13 genotype and environmental factors in the risk for allergic rhinitis in Korean children. J Allergy Clin Immunol. 2012; 130:421–426.e5.90. Sordillo JE, Sharma S, Poon A, Lasky-Su J, Belanger K, Milton DK, Bracken MB, Triche EW, Leaderer BP, Gold DR, Litonjua AA. Effects of endotoxin exposure on childhood asthma risk are modified by a genetic polymorphism in ACAA1. BMC Med Genet. 2011; 12:158.91. Panasevich S, Lindgren C, Kere J, Wickman M, Pershagen G, Nyberg F, Melen E. Interaction between early maternal smoking and variants in TNF and GSTP1 in childhood wheezing. Clin Exp Allergy. 2010; 40:458–467.92. Bieli C, Eder W, Frei R, Braun-Fahrlander C, Klimecki W, Waser M, Riedler J, von Mutius E, Scheynius A, Pershagen G, Doekes G, Lauener R, Martinez FD. PARSIFAL study group. A polymorphism in CD14 modifies the effect of farm milk consumption on allergic diseases and CD14 gene expression. J Allergy Clin Immunol. 2007; 120:1308–1315.93. Gern JE, Reardon CL, Hoffjan S, Nicolae D, Li Z, Roberg KA, Neaville WA, Carlson-Dakes K, Adler K, Hamilton R, Anderson E, Gilbertson-White S, Tisler C, Dasilva D, Anklam K, Mikus LD, Rosenthal LA, Ober C, Gangnon R, Lemanske RF Jr. Effects of dog ownership and genotype on immune development and atopy in infancy. J Allergy Clin Immunol. 2004; 113:307–314.94. Martinez FD. CD14, endotoxin, and asthma risk: actions and interactions. Proc Am Thorac Soc. 2007; 4:221–225.95. Howell WM, Holgate ST. HLA genetics and allergic disease. Thorax. 1995; 50:815–818.96. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000; 406:782–787.97. Ramasamy A, Kuokkanen M, Vedantam S, Gajdos ZK, Couto Alves A, Lyon HN, Ferreira MA, Strachan DP, Zhao JH, Abramson MJ, Brown MA, Coin L, Dharmage SC, Duffy DL, Haahtela T, Heath AC, Janson C, Kahonen M, Khaw KT, Laitinen J, Le Souef P, Lehtimaki T, Madden PA, Marks GB, Martin NG, Matheson MC, Palmer CD, Palotie A, Pouta A, Robertson CF, Viikari J, Widen E, Wjst M, Jarvis DL, Montgomery GW, Thompson PJ, Wareham N, Eriksson J, Jousilahti P, Laitinen T, Pekkanen J, Raitakari OT, O'Connor GT, Salomaa V, Jarvelin MR, Hirschhorn JN. Australian Asthma Genetics Consortium Collaborators. Genome-wide association studies of asthma in population-based cohorts confirm known and suggested loci and identify an additional association near HLA. PLoS One. 2012; 7:e44008.98. Islam T, Berhane K, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax. 2009; 64:197–202.99. Romieu I, Moreno-Macias H, London SJ. Gene by environment interaction and ambient air pollution. Proc Am Thorac Soc. 2010; 7:116–122.