Nutr Res Pract.
2016 Jun;10(3):251-258. 10.4162/nrp.2016.10.3.251.
Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- Affiliations
-
- 1Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand. pornngarm.d@cmu.ac.th
- KMID: 2342126
- DOI: http://doi.org/10.4162/nrp.2016.10.3.251
Abstract
- BACKGROUND/OBJECTIVES
Several pharmacological properties of red rice extract have been reported including anti-oxidant, anti-tumor, and reduced cancer cell invasion. This study was conducted to evaluate the anti-inflammatory effects of red rice extract on the production of inflammatory mediators in lipopolysaccharide (LPS)-induced Raw 264.7 macrophages.
MATERIALS/METHODS
Pro-inflammatory cytokines including tumor necrosis factor-α and interleukin-6 were determined by ELISA and cyclooxygenase-2 and inducible nitric oxide synthase expression was evaluated using western blot analysis. In addition, the signaling pathway controlling the inflammatory cascade such as nuclear factor kappa B (NF-κB), activator proteins-1 (AP-1), and mitogen-activated protein kinase (MAPK) was determined.
RESULTS
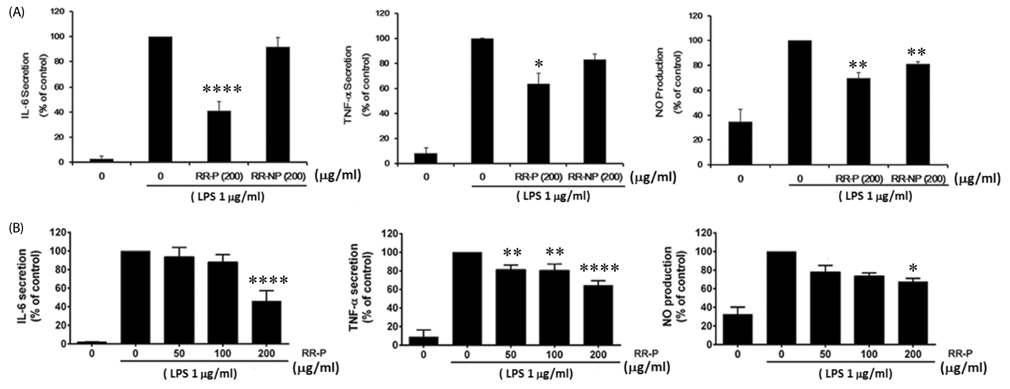
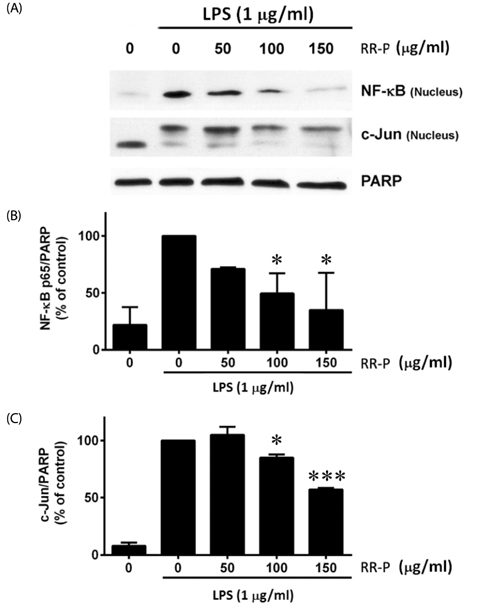
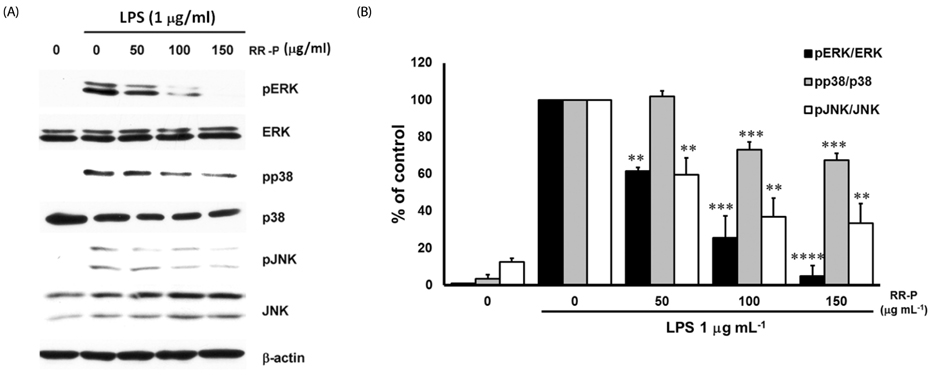
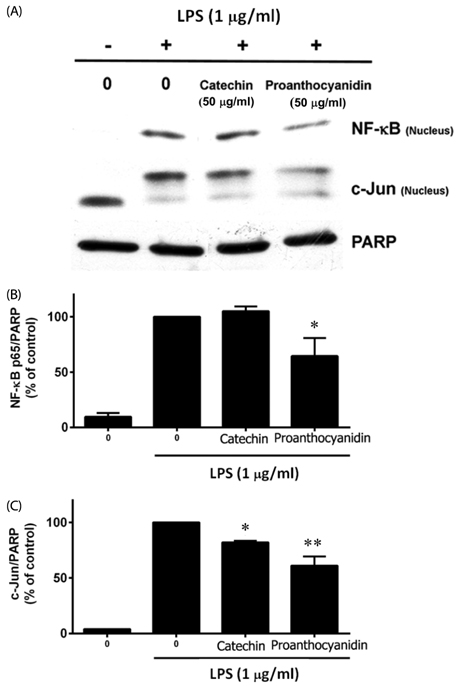
Our results showed that red rice polar extract fraction (RR-P), but not non-polar extract fraction, inhibited interleukin-6, tumor necrosis factor-α, and nitric oxide production in LPS-induced Raw 264.7 cells. RR-P also reduced the expression of inflammatory enzymes, inducible nitric oxide synthase, and cyclooxygenase-2. In addition, activation of AP-1 and NF-κB transcription factor in the nucleus was abrogated by RR-P. RR-P inhibited the phosphorylation of extracellular signaling-regulated kinase 1/2, c-Jun NH2-terminal kinase, and p38 MAPK signaling responsible for the expression of inflammatory mediators in LPS-stimulated Raw 264.7 cells. Based on chemical analysis, high amounts of proanthocyanidin and catechins were detected in the RR-P fraction. However, only proanthocyanidin reduced NF-κB and AP-1 activation in LPS-activated Raw 264.7 cells.
CONCLUSION
These observations suggest that the anti-inflammatory properties of RR-P may stem from the inhibition of pro-inflammatory mediators via suppression of the AP-1, NF-κB, and MAPKs pathways.
Keyword
MeSH Terms
-
Blotting, Western
Catechin
Cyclooxygenase 2
Cytokines
Enzyme-Linked Immunosorbent Assay
Inflammation
Interleukin-6
Macrophages*
Necrosis
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
p38 Mitogen-Activated Protein Kinases
Phosphorylation
Phosphotransferases
Protein Kinases
RAW 264.7 Cells
Transcription Factor AP-1*
Transcription Factors
Catechin
Cyclooxygenase 2
Cytokines
Interleukin-6
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphotransferases
Protein Kinases
Transcription Factor AP-1
Transcription Factors
p38 Mitogen-Activated Protein Kinases
Figure
Cited by 2 articles
-
Anti-inflammatory activities of
Scolopendra subspinipes mutilans in RAW 264.7 cells
Jae Hyeon Park, Sun Ryung Lee
J Nutr Health. 2018;51(4):323-329. doi: 10.4163/jnh.2018.51.4.323.L -Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
Zhengxuan Wang, Mingcai Liang, Hui Li, Bingxiao Liu, Lin Yang
Nutr Res Pract. 2022;16(6):729-744. doi: 10.4162/nrp.2022.16.6.729.
Reference
-
1. Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008; 214:161–178.
Article2. Watson WH, Zhao Y, Chawla RK. S-adenosylmethionine attenuates the lipopolysaccharide-induced expression of the gene for tumour necrosis factor alpha. Biochem J. 1999; 342:21–25.
Article3. Han S, Lee JH, Kim C, Nam D, Chung WS, Lee SG, Ahn KS, Cho SK, Cho M, Ahn KS. Capillarisin inhibits iNOS, COX-2 expression, and proinflammatory cytokines in LPS-induced RAW 264.7 macrophages via the suppression of ERK, JNK, and NF-kappaB activation. Immunopharmacol Immunotoxicol. 2013; 35:34–42.
Article4. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015; 160:816–827.
Article5. Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy - from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005; 1754:253–262.
Article6. Zhang Y, Yan R, Hu Y. Oxymatrine inhibits lipopolysaccharide-induced inflammation by down-regulating Toll-like receptor 4/nuclear factor-kappa B in macrophages. Can J Physiol Pharmacol. 2014; 93:253–260.
Article7. Endale M, Park SC, Kim S, Kim SH, Yang Y, Cho JY, Rhee MH. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-kappa B-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013; 218:1452–1467.
Article8. Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, Lee DU, Kim YS. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 2012; 14:375–383.
Article9. Kim GR, Jung ES, Lee S, Lim SH, Ha SH, Lee CH. Combined mass spectrometry-based metabolite profiling of different pigmented rice (Oryza sativa L.) seeds and correlation with antioxidant activities. Molecules. 2014; 19:15673–15686.
Article10. Pintha K, Yodkeeree S, Pitchakarn P, Limtrakul P. Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pac J Cancer Prev. 2014; 15:4601–4607.
Article11. Laokuldilok T, Shoemaker CF, Jongkaewwattana S, Tulyathan V. Antioxidants and antioxidant activity of several pigmented rice brans. J Agric Food Chem. 2011; 59:193–199.
Article12. Muntana N, Prasong S. Study on total phenolic contents and their antioxidant activities of Thai white, red and black rice bran extracts. Pak J Biol Sci. 2010; 13:170–174.
Article13. Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci Nutr. 2014; 2:75–104.
Article14. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010; 49:1603–1616.
Article15. Lakkur S, Judd S, Bostick RM, McClellan W, Flanders WD, Stevens VL, Goodman M. Oxidative stress, inflammation, and markers of cardiovascular health. Atherosclerosis. 2015; 243:38–43.
Article16. Wang JS, Wu D, Huang DY, Lin WW. TAK1 inhibition-induced RIP1-dependent apoptosis in murine macrophages relies on constitutive TNF-alpha signaling and ROS production. J Biomed Sci. 2015; 22:76.17. Choudhury S, Ghosh S, Gupta P, Mukherjee S, Chattopadhyay S. Inflammation-induced ROS generation causes pancreatic cell death through modulation of Nrf2/NF-kappaB and SAPK/JNK pathway. Free Radic Res. 2015; 49:1371–1383.
Article18. Mizushina Y, Kuriyama I, Yamazaki A, Akashi T, Yoshida H. Cycloartenyl trans-ferulate, a component of the bran byproduct of sake-brewing rice, inhibits mammalian DNA polymerase and suppresses inflammation. Food Chem. 2013; 141:1000–1007.
Article19. Ng LT, Ko HJ. Comparative effects of tocotrienol-rich fraction, alpha-tocopherol and alpha-tocopheryl acetate on inflammatory mediators and nuclear factor kappa B expression in mouse peritoneal macrophages. Food Chem. 2012; 134:920–925.
Article20. Lyu SY, Park WB. Production of cytokine and NO by and PBMC in vitro incubation with RAW 264.7 macrophages flavonoids. Arch Pharm Res. 2005; 28:573–581.
Article21. Ronchetti D, Borghi V, Gaitan G, Herrero JF, Impagnatiello F. NCX 2057, a novel NO-releasing derivative of ferulic acid, suppresses inflammatory and nociceptive responses in in vitro and in vivo models. Br J Pharmacol. 2009; 158:569–579.
Article22. Ahmad SF, Zoheir KM, Abdel-Hamied HE, Ashour AE, Bakheet SA, Attia SM, Abd-Allah AR. Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. Int Immunopharmacol. 2013; 17:79–87.
Article23. Zhang H, Shao Y, Bao J, Beta T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015; 172:630–639.
Article24. Gunaratne A, Wu K, Li D, Bentota A, Corke H, Cai YZ. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013; 138:1153–1161.
Article25. Wongsirisin P, Yodkeeree S, Pompimon W, Limtrakul P. Induction of G1 arrest and apoptosis in human cancer cells by crebanine, an alkaloid from Stephania venosa. Chem Pharm Bull (Tokyo). 2012; 60:1283–1289.
Article26. Lee J, Nakagiri T, Kamimura D, Harada M, Oto T, Susaki Y, Shintani Y, Inoue M, Miyoshi S, Morii E, Hirano T, Murakami M, Okumura M. IL-6 amplifier activation in epithelial regions of bronchi after allogeneic lung transplantation. Int Immunol. 2013; 25:319–332.
Article27. Kwon DJ, Ju SM, Youn GS, Choi SY, Park J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-kappaB and AP-1 activation in RAW 264.7 macrophages. Food Chem Toxicol. 2013; 58:479–486.
Article28. Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009; 69:6581–6589.
Article29. Choi RJ, Chun J, Khan S, Kim YS. Desoxyrhapontigenin, a potent anti-inflammatory phytochemical, inhibits LPS-induced inflammatory responses via suppressing NF-kappaB and MAPK pathways in RAW 264.7 cells. Int Immunopharmacol. 2014; 18:182–190.
Article30. Lu Y, Suh SJ, Kwak CH, Kwon KM, Seo CS, Li Y, Jin Y, Li X, Hwang SL, Kwon O, Chang YC, Park YG, Park SS, Son JK, Kim CH, Chang HW. Saucerneol F, a new lignan, inhibits iNOS expression via MAPKs, NF-kappaB and AP-1 inactivation in LPS-induced RAW264.7 cells. Int Immunopharmacol. 2012; 12:175–181.
Article31. Kwon JY, Hong SH, Park SD, Ahn SG, Yoon JH, Kwon BM, Kim SA. 2'-Benzoyloxycinnamaldehyde inhibits nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells via regulation of AP-1 pathway. Eur J Pharmacol. 2012; 696:179–186.
Article32. Khan S, Shehzad O, Lee KJ, Tosun A, Kim YS. Anti-inflammatory properties of samidin from Seseli resinosum through suppression of NF-kappa B and AP-1-mediated-genes in LPS-stimulated RAW 264.7 cells. Arch Pharm Res. 2014; 37:1496–1503.
Article33. Jang S, Xu Z. Lipophilic and hydrophilic antioxidants and their antioxidant activities in purple rice bran. J Agric Food Chem. 2009; 57:858–862.
Article34. Jun HI, Song GS, Yang EI, Youn Y, Kim YS. Antioxidant activities and phenolic compounds of pigmented rice bran extracts. J Food Sci. 2012; 77:C759–C764.
Article35. Burdette A, Garner PL, Mayer EP, Hargrove JL, Hartle DK, Greenspan P. Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J Med Food. 2010; 13:879–887.
Article36. Inglett GE, Chen D, Rose DJ, Berhow M. High-shear, jet-cooking, and alkali treatment of corn distillers' dried grains to obtain products with enhanced protein, oil and phenolic antioxidants. Food Sci Technol Int. 2010; 16:297–304.
Article37. Srisaipet A, Nuddagul M. Influence of temperature on gamma-oryzanol stability of edible rice bran oil during heating. Int J Chem Eng Appl. 2014; 5:303–306.
Article38. Cuevas-Rodríguez EO, Dia VP, Yousef GG, García-Saucedo PA, López-Medina J, Paredes-López O, Gonzalez de, Lila MA. Inhibition of pro-inflammatory responses and antioxidant capacity of Mexican blackberry (Rubus spp.) extracts. J Agric Food Chem. 2010; 58:9542–9548.
Article39. Kim IS, Park YJ, Yoon SJ, Lee HB. Ephedrannin A and B from roots of Ephedra sinica inhibit lipopolysaccharide-induced inflammatory mediators by suppressing nuclear factor-kappa B activation in RAW 264.7 macrophages. Int Immunopharmacol. 2010; 10:1616–1625.
Article40. Chalermpong S. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J Med Plant Res. 2012; 6:1070–1077.41. Qureshi AA, Reis JC, Papasian CJ, Morrison DC, Qureshi N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis. 2010; 9:143.
Article42. Wang Y, Jiang Q. gamma-Tocotrienol inhibits lipopolysaccharideinduced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPbeta and NF-kappaB in macrophages. J Nutr Biochem. 2013; 24:1146–1152.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- Aloe-emodin inhibits Pam₃CSK₄-induced MAPK and NF-κB signaling through TLR2 in macrophages
- Estragole Exhibits Anti-inflammatory Activity with the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-induced RAW 264.7 cells
- Anti-inflammatory Activity of Standardized Fraction from Inula helenium L. via Suppression of NF-κB Pathway in RAW 264.7 Cells
- Pulegone Exhibits Anti-inflammatory Activities through the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-stimulated RAW 264.7 cells