Nutr Res Pract.
2013 Apr;7(2):96-102.
Inhibitory effects of Capsicum annuum L. water extracts on lipoprotein lipase activity in 3T3-L1 cells
- Affiliations
-
- 1Divison of Bio-Health Technology, College of Biomedical Science, Kangwon National University, 192-1 Hyoja-dong, Chuncheon-si, Gangwon 200-701, Korea. mchoe@kangwon.ac.kr
- 2Well-Being Bioproducts RIC Center, Kangwon National University, Gangwon 225-874, Korea.
- 3Enzymebio Co., LTD, Chuncheon, Gangwon 200-870, Korea.
- 4Kitami Institute of Technology, 165 Koen-cho, Kitami 090-8507, Japan.
Abstract
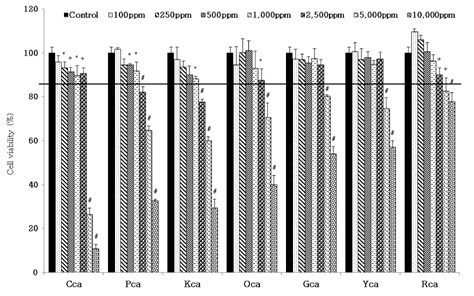
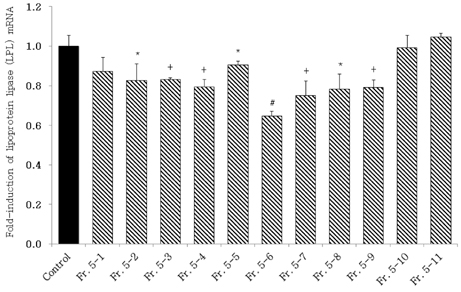
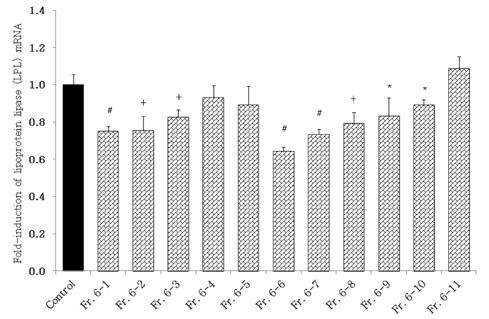
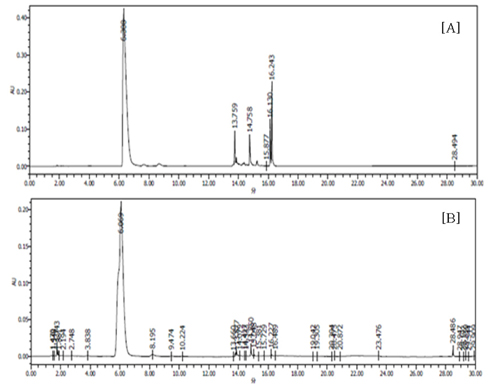
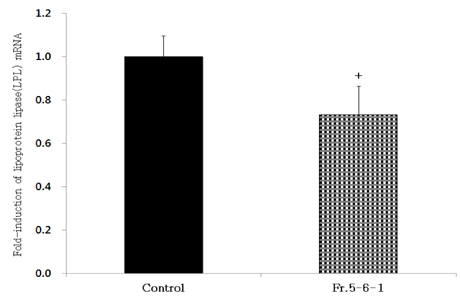
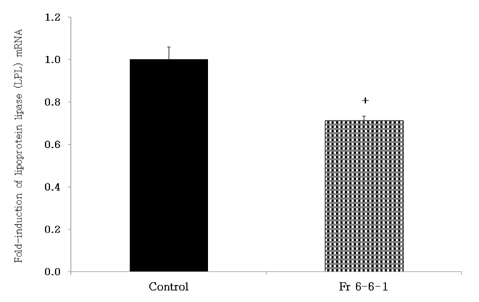
- Obesity, an intractable metabolic disease, currently has no medical treatment without side effects, so studies have been actively carried out to find natural compounds that have anti-obesity activity with minimum side effects. In this study, the anti-obesity effects of water extracts of seven Capsicum annuum L. varieties being Putgochu (Pca), Oyee gochu (Oca), Kwari putgochu (Kca), Green pepper (Gca), Yellow paprika (Yca), Red paprika (Rca) and Cheongyang gochu (Cca), were examined through the evaluation of lipoprotein lipase (LPL) mRNA expression level in 3T3-L1 cells (mouse pre-adipocytes). After capsaicin elimination by chloroform defatting, freeze-dried powder of Cca was treated to 3T3-L1 cells and anti-obesity effects were examined by determining the LPL mRNA level using the RT-PCR method. Of the primary fractions, only proven fractions underwent secondary and tertiary refractionating to determine anti-obesity effects. From seven different Capsicum annuum L., there was a significant decrease of the LPL mRNA expression level of 50.9% in Cca treatment compared to the control group. A significant decrease of the LPL mRNA expression level was shown in primary fractions (Fr) 5 (36.2% decrease) and 6 (30.5% decrease) of the Cca water extracts. Due to the impurities checked by UPLC chromatography, Fr 5 and 6 were refractionated to determine the LPL mRNA expression level. Treatment of Fr 6-6 (35.8% decrease) and Fr 5-6 (35.3% decrease) showed a significant decrease in the LPL mRNA expression level. When analyzed using UPLC, major compounds of Fr 6-6 and Fr 5-6 were very similar. Subsequently, we refractionated Fr 6-6 and Fr 5-6 to isolate the major peak for structure elucidation. Treatment of Fr 5-6-1 (26.6% decrease) and Fr 6-6-1 (29.7% decrease) showed a significant decrease in the LPL mRNA expression level. Consequently, the fractions may have a possibility to ameliorate obesity through the decrease of the LPL mRNA expression level.
MeSH Terms
Figure
Reference
-
1. Bray MS. Genomics, genes, and environmental interaction: the role of exercise. J Appl Physiol. 2000. 88:788–792.2. Nam HK, Cho JH, Kim SH. Relations between self-selected intake of nutrients and body fat accumulation in rats fed ad libitum or for 8-hours a day. Korean J Nutr. 1992. 25:12–21.3. Huh KB. Symposium: recent progress in obesity research: pathogenesis of obesity. Korean J Nutr. 1990. 23:333–336.4. Sjöström LV, Lönn L, Chowdhury B, Grangård U, Lissner L, Sjöström D, Sullivan L. Angel A, Anderson H, Lau BD, Leiter L, Mendelson R, editors. The sagittal diameter is a valid maker of the visceral adipose tissue volume. Progress in Obesity Research: 7. 1996. London: John Libbey & Co. Ltd.;309–319.5. Sjöström LV. Morbidity of severely obese subjects. Am J Clin Nutr. 1992. 55:508S–515S.
Article6. Kim SG, An GH, Yoon SW, Lee YC, Ha SD. A study on dietary supplement to reduce obesity by the mechanism of decreasing lipid and carbohydrate absorption. Korean J Food Sci Technol. 2003. 35:519–526.7. Vivero LE, Anderson PO, Clark RF. A close look at fenfluramine and dexfenfluramine. J Emerg Med. 1998. 16:197–205.
Article8. Park HS. Pharmacological therapy of obesity. J Korean Soc Study Obes. 2001. 10:118–127.9. Kim YS. Drug treatment of obesity. J Korean Soc Endocrinol. 2001. 16:9–15.10. Chang SH, Jun MH, Kang TB, Mun SH, Lee JH, Seong NS, Lee ST, Kim JB, Her E. The effect of Korean mistletoe extract M11C (non-lectin components) on IL-1β release and expression from macrophages. Immune Netw. 2001. 1:170–178.
Article11. Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004. 96:229–245.
Article12. Chang CH. The future prospect of traditional Korean fermented foods. Korean J Diet Cult. 1988. 3:341–345.13. Sambaiah K, Satyanarayana MN. Influence of red pepper and capsaicin on body composition and lipogenesis in rats. J Biosci. 1982. 4:425–430.
Article14. Watanabe T, Kawada T, Iwai K. Enhancement by capsaicin of energy metabolism in rats through secretion of catecholamine from adrenal medulla. Agric Biol Chem. 1987. 51:75–79.
Article15. Chae KY, Hong JS. The quality characteristics of Jeolpyon with different amounts of job's tears flour. Korean J Food Cookery Sci. 2007. 23:770–776.16. Buck SH, Burks TF. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986. 38:179–226.17. Hsu CL, Yen GC. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J Agric Food Chem. 2007. 55:1730–1736.
Article18. Watanabe T, Kawada T, Yamamoto M, Iwai K. Capsaicin, a pungent principle of hot red pepper, evokes catecholamine secretion from the adrenal medulla of anesthetized rats. Biochem Biophys Res Commun. 1987. 142:259–264.
Article19. Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr. 1986. 116:1272–1278.
Article20. Srinivasan MR, Satyanarayana MN. Effect of capsaicin on skeletal muscle lipoprotein lipase in rats fed high fat diet. Indian J Exp Biol. 1989. 27:910–912.21. Lee HJ. Studies on effects of natural plants water extracts on activities of enzymes related with fat metabolism [master's thesis]. 2009. Chuncheon: Kangwon National University.22. Seo DJ. Anti-obesity effects of natural plants mixture in high fat diet-induced C57BL/6J mice [master's thesis]. 2010. Chuncheon: Kangwon National University.23. Henderson DE, Slickman AM, Henderson SK. Quantitative HPLC determination of the antioxidant activity of capsaicin on the formation of lipid hydroperoxides of linoleic acid: a comparative study against BHT and melatonin. J Agric Food Chem. 1999. 47:2563–2570.
Article24. Kempaiah RK, Manjunatha H, Srinivasan K. Protective effect of dietary capsaicin on induced oxidation of low-density lipoprotein in rats. Mol Cell Biochem. 2005. 275:7–13.
Article25. Malmberg AB, Mizisin AP, Calcutt NA, von Stein T, Robbins WR, Bley KR. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004. 111:360–367.
Article26. Surh YJ, Lee RC, Park KK, Mayne ST, Liem A, Miller JA. Chemoprotective effects of capsaicin and diallyl sulfide against mutagenesis or tumorigenesis by vinyl carbamate and N-nitrosodimethylamine. Carcinogenesis. 1995. 16:2467–2471.
Article27. Reddy AC, Lokesh BR. Studies on anti-inflammatory activity of spice principles and dietary n-3 polyunsaturated fatty acids on carrageenan-induced inflammation in rats. Ann Nutr Metab. 1994. 38:349–358.
Article28. Jeon G, Choi Y, Lee SM, Kim Y, Jeong HS, Lee J. Anti-obesity activity of methanol extract from hot pepper (Capsicum annuum L.) seeds in 3T3-L1 adipocyte. Food Sci Biotechnol. 2010. 19:1123–1127.
Article29. Lee JJ, Choi MS, Chung CS, Choi BD. Effects of food restriction on rat adipose tissue lipoprotein lipase activity and lipogenesis. Korean J Exerc Nutr. 2003. 7:135–141.30. Lee SK, Kim OS, Kim JY, Lee KH, Baik HW, Jo YS, Kim HJ, Kim BJ, Park KS, Ahn KJ. Lipoprotein lipase hind 3 polymorphism, blood lipid levels and obesity in Korean women. 2007. In : 2007 Annual Meeting of Korean Endocrine Society; 2007 May 3-4; Seoul, Korea. Seoul: Korean Endocrine Society;133.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of Capsicum annuum L. water extracts on lipoprotein lipase activity in 3T3-L1 cells
- Effect of Dexamethasone, Retinoid Acid and Vitamin D on the Transdifferentiation of 3T3-L1 and KS483
- Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- In Vitro α-Amylase, α-Glucosidase, Pancreatic Lipase, Xanthine Oxidase Inhibiting Activity of Agaricus bisporus Extracts