Nutr Res Pract.
2013 Feb;7(1):34-42.
Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in STZ-induced rats
- Affiliations
-
- 1Department of Food Science and Nutrition, Daedeok Valley Campus, Hannam University, 461-6 Jeonmin-dong, Yuseng-gu, Deajeon 305-811, Korea. mhkang@hnu.kr
Abstract
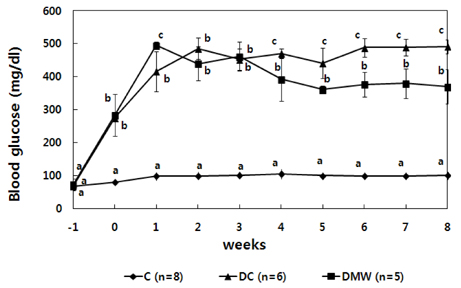
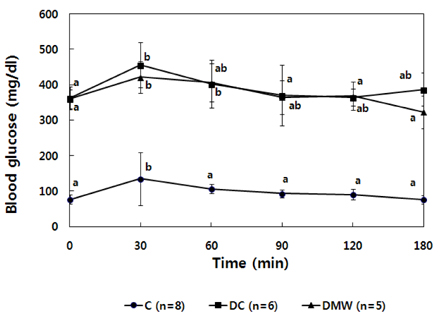
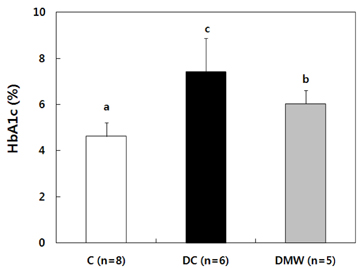
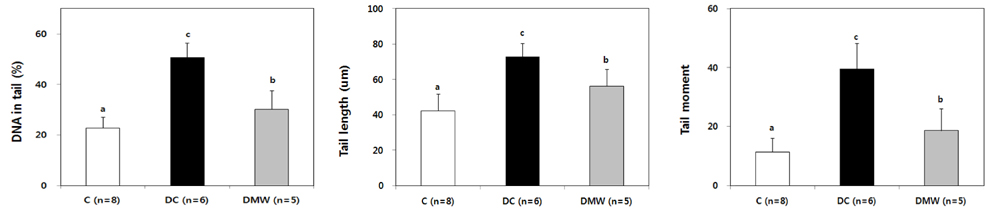
- This study investigated the effects of magnetized water supplementation on blood glucose, DNA damage, antioxidant status, and lipid profiles in streptozotocin (STZ)-induced diabetic rats. There were three groups of 4-week-old male Sprague-Dawley rats used in the study: control group (normal control group without diabetes); diabetes group (STZ-induced diabetes control); and magnetized water group (magnetized water supplemented after the induction of diabetes using STZ). Before initiating the study, diabetes was confirmed by measuring fasting blood glucose (FBS > 200 dl), and the magnetized water group received magnetized water for 8 weeks instead of general water. After 8 weeks, rats were sacrificed to measure the fasting blood glucose, insulin concentration, glycated hemoglobin level, degree of DNA damage, antioxidant status, and lipid profiles. From the fourth week of magnetized water supplementation, blood glucose was decreased in the magnetized water group compared to the diabetes group, and such effect continued to the 8th week. The glycated hemoglobin content in the blood was increased in the diabetes group compared to the control group, but decreased significantly in the magnetized water group. However, decreased plasma insulin level due to induced diabetes was not increased by magnetized water supplementation. Increased blood and liver DNA damages in diabetes rats did significantly decrease after the administration of magnetized water. In addition, antioxidant enzyme activities and plasma lipid profiles were not different among the three groups. In conclusion, the supplementation of magnetized water not only decreased the blood glucose and glycated hemoglobin levels but also reduced blood and liver DNA damages in STZ-induced diabetic rats. From the above results, it is suggested that the long-term intake of the magnetized water over 8 weeks may be beneficial in both prevention and treatment of complications in diabetic patients.
MeSH Terms
Figure
Reference
-
1. Statistics Korea [Internet]. 2010. Daejeon: Statistics Korea;Available from: http://www.kostat.go.kr.2. Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 1999. 3rd ed. New York: Oxford University Press;20–37.3. Feillet-Coudray C, Rock E, Coudray C, Grzelkowska K, Azais-Braesco V, Dardevet D, Mazur A. Lipid peroxidation and antioxidant status in experimental diabetes. Clin Chim Acta. 1999. 284:31–43.
Article4. West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000. 17:171–180.
Article5. Collins AR, Raslová K, Somorovská M, Petrovská H, Ondrusová A, Vohnout B, Fábry R, Dusinská M. DNA damage in diabetes: correlation with a clinical marker. Free Radic Biol Med. 1998. 25:373–377.
Article6. Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, Nicotera T. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996. 347:444–445.
Article7. Hinokio Y, Suzuki S, Hirai M, Chiba M, Hirai A, Toyota T. Oxidative DNA damage in diabetes mellitus: its association with diabetic complications. Diabetologia. 1999. 42:995–998.
Article8. Park KS, Kim JH, Kim MS, Kim JM, Kim SK, Choi JY, Chung MH, Han B, Kim SY, Lee HK. Effects of insulin and antioxidant on plasma 8-hydroxyguanine and tissue 8-hydroxydeoxyguanosine in streptozotocin-induced diabetic rats. Diabetes. 2001. 50:2837–2841.
Article9. Ramon O, Wong HK, Joyeux M, Riondel J, Halimi S, Ravanat JL, Favier A, Cadet J, Faure P. 2'-deoxyguanosine oxidation is associated with decrease in the DNA-binding activity of the transcription factor Sp1 in liver and kidney from diabetic and insulin-resistant rats. Free Radic Biol Med. 2001. 30:107–118.
Article10. Mahesh T, Menon VP. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res. 2004. 18:123–127.
Article11. Young IS, Torney JJ, Trimble ER. The effect of ascorbate supplementation on oxidative stress in the streptozotocin diabetic rat. Free Radic Biol Med. 1992. 13:41–46.
Article12. Suhail M, Patil S, Khan S, Siddiqui S. Antioxidant vitamins and lipoperoxidation in non-pregnant, pregnant, and gestational diabetic women: erythrocytes osmotic fragility profiles. J Clin Med Res. 2010. 2:266–273.
Article13. Shidfar F, Homayounfar R, Fereshtehnejad SM, Kalani A. Effect of folate supplementation on serum homocysteine and plasma total antioxidant capacity in hypercholesterolemic adults under lovastatin treatment: a double-blind randomized controlled clinical trial. Arch Med Res. 2009. 40:380–386.
Article14. Roussel AM, Kerkeni A, Zouari N, Mahjoub S, Matheau JM, Anderson RA. Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J Am Coll Nutr. 2003. 22:316–321.
Article15. Yu YM, Chang WC, Chang CT, Hsieh CL, Tsai CE. Effects of young barley leaf extract and antioxidative vitamins on LDL oxidation and free radical scavenging activities in type 2 diabetes. Diabetes Metab. 2002. 28:107–114.16. Kim HY, Jeon EJ, Park YK, Kang MH. Effect of deer antler drink supplementation on blood pressure, blood glucose and lymphocyte DNA damage in type 2 diabetic patients. Korean J Nutr. 2004. 37:794–800.17. Lee SH, Lee HJ, Lee YH, Lee BW, Cha BS, Kang ES, Ahn CW, Park JS, Kim HJ, Lee EY, Lee HC. Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague-Dawley rats. Phytother Res. 2012. 26:142–147.
Article18. Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J Am Coll Nutr. 2009. 28:355–361.
Article19. Ma YL, Ren H, Ren S, Zhen EK, Hao G, Zhao YW. A study of the effect of magnetized water on enzyme activities by potentiometric enzyme electrode method. J Tongji Med Univ. 1992. 12:193–196.
Article20. Johnson KE, Sanders JJ, Gellin RG, Palesch YY. The effectiveness of a magnetized water oral irrigator (Hydro Floss) on plaque, calculus and gingival health. J Clin Periodontol. 1998. 25:316–321.
Article21. Zhang YS, Wu HW. Effect of magnetic water on urinary calculi--an experimental and clinical study. Z Urol Nephrol. 1987. 80:517–523.22. Zhang YS, Wu HW. Effect of magnetized water on urinary calculi: an experimental and clinical study. Acta Acad Med Wuhan. 1984. 4:31–37.23. Lee HJ, Jo HR, Jeon EJ, Kang MH. Effect of the magnetized water supplementation on lymphocyte DNA damage in mice treated with diethylnitrosamine. Korean J Nutr. 2010. 43:570–577.
Article24. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article25. Lee HJ, Park YK, Kang MH. The effect of carrot juice, β-carotene supplementation on lymphocyte DNA damage, erythrocyte antioxidant enzymes and plasma lipid profiles in Korean smoker. Nutr Res Pract. 2011. 5:540–547.
Article26. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988. 175:184–191.
Article27. Jeon SI, Kim DR, Lee SK. Changes of solubility speed of salts in magnetized water and crystal patterns of NaCl, KCl and gypsom intermediated by magnetized water. J Korean Chem Soc. 2001. 45:116–130.28. Wu J. Further observations on the therapeutic effect of magnets and magnetized water against ascariasis in children--analysis of 114 cases. J Tradit Chin Med. 1989. 9:111–112.29. Watt DL, Rosenfelder C, Sutton CD. The effect of oral irrigation with a magnetic water treatment device on plaque and calculus. J Clin Periodontol. 1993. 20:314–317.
Article30. Xu YB, Sun SY. Effect of stable weak magnetic field on Cr(VI) bio-removal in anaerobic SBR system. Biodegradation. 2008. 19:455–462.
Article31. Gonet B. Influence of constant magnetic fields on certain physiochemical properties of water. Bioelectromagnetics. 1985. 6:169–175.
Article32. Lednev VV. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics. 1991. 12:71–75.
Article33. Liboff AR, Cherng S, Jenrow KA, Bull A. Calmodulin-dependent cyclic nucleotide phosphodiesterase activity is altered by 20 μT magnetostatic fields. Bioelectromagnetics. 2003. 24:32–38.
Article34. Kang BJ. Magnetized Water. 2005. Seoul: Seoum Media Publishing Co;49–63.35. Kim MJ, Jung KH, Uhm YK, Leem KH, Kim HK. Preservative effect of electrolyzed reduced water on pancreatic beta-cell mass in diabetic db/db mice. Biol Pharm Bull. 2007. 30:234–236.
Article36. Radin D, Hayssen G, Emoto M, Kizu T. Double-blind test of the effects of distant intention on water crystal formation. Explore (NY). 2006. 2:408–411.
Article37. Emoto M. Healing with water. J Altern Complement Med. 2004. 10:19–21.
Article38. Merkulova IU, Mikhel'son ML. The therapeutic action of magnetized water on the quality of health at a sanatorium. Vopr Kurortol Fizioter Lech Fiz Kult. 1994. 2:37–39.39. Mikhel'son ML, Merkulova IU. The biological and bactericidal actions of magnetized water. Vopr Kurortol Fizioter Lech Fiz Kult. 1994. 2:35–37.40. Zhang JG. Preventing bacillary dysentery with magnetized drinking water. Zhonghua Liu Xing Bing Xue Za Zhi. 1985. 6:203–205.41. Lucchetti G, Lucchetti AL, Bassi RM, Nobre MR. Complementary spiritist therapy: systematic review of scientific evidence. Evid Based Complement Alternat Med. 2011. 2011:835945.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in STZ-induced rats
- Effect of the Magnetized Water Supplementation on Lymphocyte DNA Damage in Mice Treated with Diethylnitrosamine
- Four months of magnetized water supplementation improves glycemic control, antioxidant status, and cellualr DNA damage in db/db mice
- The Improvement of Chaga Mushroom (Inonotus Obliquus) Extract Supplementation on the Blood Glucose and Cellular DNA Damage in Streptozotocin-Induced Diabetic Rats
- Effects of Araliaceae Water Extracts on Blood Glucose Level and Biochemical Parameters in Diabetic Rats