Nutr Res Pract.
2010 Feb;4(1):16-22.
Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts
- Affiliations
-
- 1Department of Bioscience and Biotechnology, Silla University, San 1-1, Kwaebop-dong, Sasang-gu, Busan 617-736, Korea. slee@silla.ac.kr
- 2Department of Food and Nutrition, Silla University, Busan 617-736, Korea.
- 3Bioport Korea Co., Marine Bio-industry Development Center, Busan 619-912, Korea.
Abstract
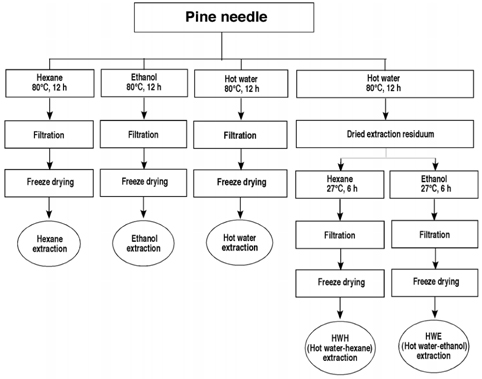
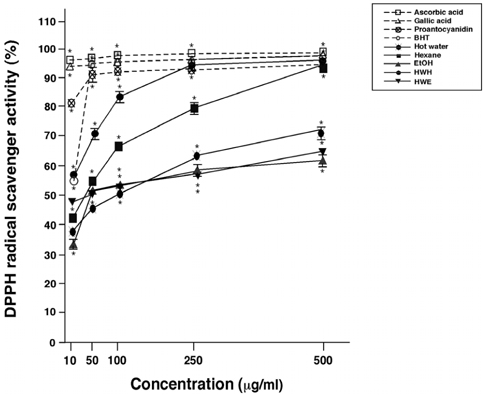
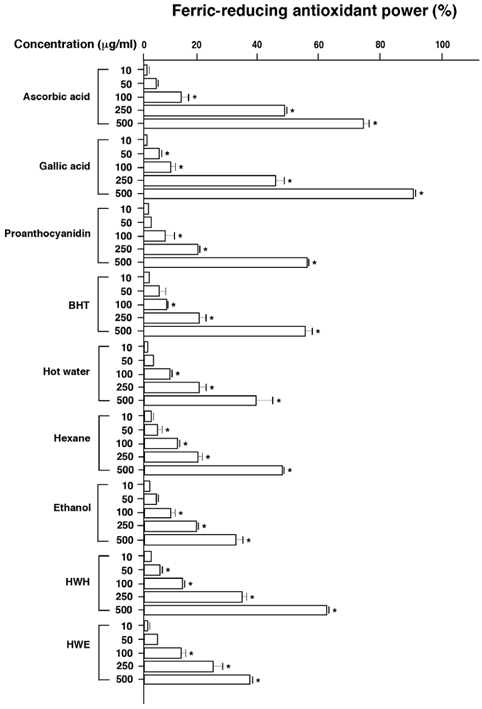
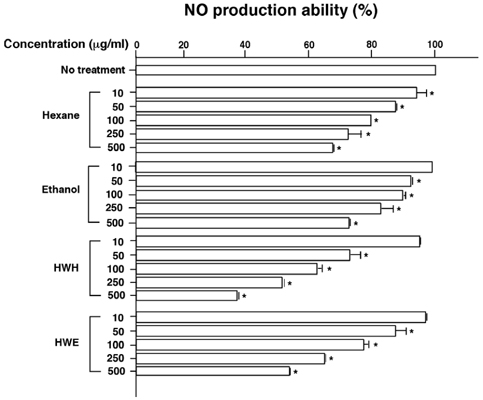
- Flavonoids are known to be effective scavengers of free radicals. In particular, proanthocyanidins are flavonoids that possess cardiovascular protection, antioxidative activities, and immunomodulatory activities. Here, we evaluated proanthocyanidin contents in the total polyphenolic compounds of pine needle extracts prepared by hot water, ethanol, hexane, hot water-hexane (HWH), and hot water-ethanol (HWE). Analysis of each extract indicated that the ethanol extract contained the highest proanthocyanidin concentration. The HWH and hexane extracts also contained relatively high concentrations of proanthocyanidin. On the other hand, proanthocyanidin content analyses out of the total polyphenolic compounds indicated that the HWH extract contained the highest content. These results suggest that HWH extraction is a suitable method to obtain an extract with a high level of pure proanthocyanidins and a relatively high yield. The HWH extract possessed superior activity in diverse antioxidative analyses such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferrous ion chelating (FIC), and ferric-ion reducing power (FRAP) assays. In addition, upon assessing the effects of the pine needle extracts on macrophages (Raw 264.7 cell), the HWH extract exhibited the highest activity. In this study, we discerned an efficient extraction method to achieve relatively pure proanthocyanidins from pine needles and evaluated the biological functions of the resulting extract, which could potentially be used for its efficacious components in functional food products.
MeSH Terms
Figure
Reference
-
1. Liu YZ, Cao YG, Ye JQ, Wang WG, Song KJ, Wang XL, Wang CH, Li RT, Deng XM. Immunomodulatory effects of proanthocyanidin A-1 derived in vitro from Rhododendron spiciferum. Fitoterapia. 2009. 81:108–114.
Article2. Ku CS, Sathishkumar M, Mun SP. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere. 2007. 67:1618–1627.
Article3. Porter LJ. Harborne JB, editor. The Flavonoids. Advances in Research since 1986. 1986. London: Chapman & Hall;23–55.4. Bagchi M, Mimes M, Williams C, Balmoori J, Ye X, Stohs S, Bagchi D. Acute and chronic stress-induced oxidative gastrointestinal injury in rats and the protective ability of a novel grape seed proanthocyanidin extract. Nutr Res. 1999. 19:1189–1199.
Article5. Bagchi D, Garg A, Krohn RL, Bagchi M, Bagchi DJ, Balmoori J, Stohs SJ. Protective effects grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol. 1998. 30:771–776.
Article6. Wang D, Chen J, Xu Z, Qiao X, Huang L. Disappearance of polycyclic aromatic hydrocarbons sorbed on surfaces of pine [Pinua thunbergii] needles under irradiation of sunlight: Volatilization and photolysis. Atmos Environ. 2005. 39:4583–4591.
Article7. Jeong JB, Seo EW, Jeong HJ. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food Chem Toxicol. 2009. 47:2135–2141.
Article8. Kim KY, Chung HJ. Flavor compounds of pine sprout tea and pine needle tea. J Agric Food Chem. 2000. 48:1269–1272.
Article9. Yu L, Zhao M, Wang JS, Cui C, Yang B, Jiang Y, Zhao Q. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb) bark. Inno Food Sic & Emer Tech. 2009. 9:122–128.
Article10. Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999. 47:3954–3962.
Article11. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995. 28:25–30.
Article12. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996. 239:70–76.
Article13. Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavenges. Arch Biochem Biophys. 1994. 315:161–169.
Article14. Duan XW, Jiang YM, Su XG, Zhang ZQ, Shi J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2006. 101:1365–1371.
Article15. Lee JC, Lim KT, Jang YS. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim Biophys Acta. 2002. 1570:181–191.
Article16. Shih PW, Lai PL, Jen HWK. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006. 99:775–783.
Article17. Frankel EN. Recent advances in lipid oxidation. J Agric Food Chem. 1991. 54:495–511.18. Gordon MH. Hudson BJF, editor. The mechanism of the antioxidant action in vitro. Food Antioxidants. 1990. London: Elsevier;1–18.19. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982. 126:131–138.20. Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baumii extracts. Food Chem. 2003. 82:593–597.
Article21. Gardner PT, White TC, McPhail DB, Duthie GG. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000. 68:471–474.
Article22. Miller DD. Fennema OR, editor. Mineral. Food Chemistry. 1996. New York: Marcel Dekker;618–649.23. Dalbo S, Jurgensen S, Horst H, Soethe DN, Santos ARS, Pizzolatti MG, Valle RMR. Analysis of the antinociceptive effect of the proanthocyanidin-rich fraction obtained from Croton Celtidifolius barks: evidence for a role of the dopaminergic system. Pharmacol Biochem Behav. 2006. 85:317–323.
Article24. Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005. 289:R771–R775.
Article25. Preuss HG, Wallerstedt D, Talpur N, Tutuncuoglu SO, Echard B, Myers A, Bui M, Bagchi D. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: a pilot study. J Med. 2000. 31:227–246.26. Sato M, Bagchi D, Tosaki A, Das DK. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic Biol Med. 2001. 31:729–737.
Article27. Uchida S, Hirai K, Hatanaka J, Hanato J, Umegaki K, Yamada S. Antinociceptive effects of St. John's wort, Harpagophytum procumbens extract and grape seed proanthocyanidins extract in mice. Biol Pharm Bull. 2008. 31:240–245.
Article28. Shukla S, Mehta A, John J, Singh S, Mehta P, Vyas SP. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food Chem Toxicol. 2009. 47:1848–1857.29. Ismail HI, Chan KW, Mariod AA, Ismail M. Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts. Food Chem. 2010. 119:643–647.
Article30. Baydar NG, Özkan G, Sağdiç O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control. 2004. 15:335–339.
Article31. Souque JM, Cheynier VR, Brossaud F, Moutounet M. Polymeric proanthocyanidins from grape skins. Phytochemisty. 1996. 43:509–512.
Article32. Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001. 49:4619–4626.
Article33. Evelsona P, Travacioa M, Repettoa M. Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys. 2001. 388:261–266.
Article34. Van den Berg R, Haenenb GRMM, Van den Berga H. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999. 66:511–517.
Article35. Halliwell B, Gutteridge JMC. Halliwell B, Gutteridge JMC, editors. Antioxidant defences. Free Radicals in Biology and Medicine. 1999. New York: Oxford University Press;200–216.36. Kunkel SL. Th1- and Th2-type cytokines regulate chemokine expression. Biol Signals. 1996. 5:197–202.
Article37. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003. 54:469–487.38. Manthey CL, Perera PY, Salkowski CA, Vogel SN. Taxol provides a second signal for murine macrophage tumoricidal activity. J Immunol. 1994. 152:825–831.39. Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996. 51:383–394.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of methods for proanthocyanidin extraction from pine (Pinus densiflora) needles and biological activities of the extracts
- Antioxidant activity and analysis of proanthocyanidins from pine (Pinus densiflora) needles
- Effects of Ectomycorrhizal Fungi on Growth of Seedlings of Pinus densiflora
- Ectomycorrhizal Fungal Communities of Red Pine (Pinus densiflora) Seedlings in Disturbed Sites and Undisturbed Old Forest Sites
- Two Species of Endophytic Cladosporium in Pine Trees in Korea