Nutr Res Pract.
2007 Sep;1(3):184-188.
Inhibitory activity of Euonymus alatus against alpha-glucosidase in vitro and in vivo
- Affiliations
-
- 1Biohealth Product Research Center, School of Food and Life Science, Institute for Food Sciences, Institute of Biomedical Engineering, Inje University, Gimhae, Gyungnam 621-749, Korea. fdsnkiji@inje.ac.kr
Abstract
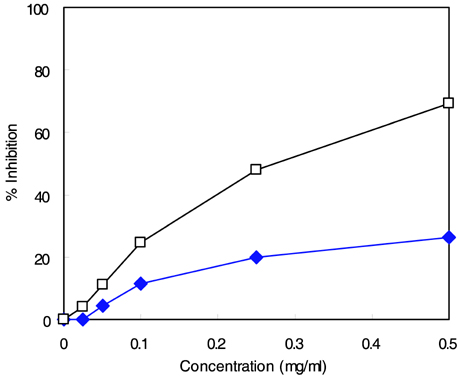
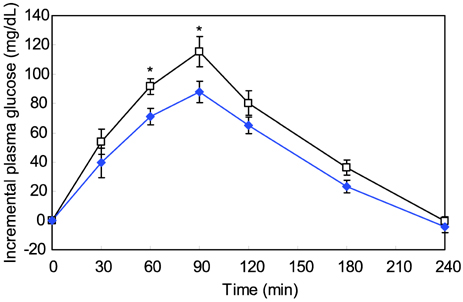
- The major goal in the treatment of diabetes mellitus is to achieve near-normal glycemic control. To optimize both fasting blood glucose and postprandial glucose levels is important in keeping blood glucose levels as close to normal as possible. alpha-Glucosidase is the enzyme that digests dietary carbohydrate, and inhibition of this enzyme could suppress postprandial hyperglycemia. The purpose of this study was to test the inhibitory activity of methanol extract of Euonymus alatus on alpha-glucosidase in vitro and in vivo to evaluate its possible use as an anti-diabetic agent. Yeast alpha-glucosidase inhibitory activities of methanol extract of E. alatus were measured at concentrations of 0.50, 0.25, 0.10, and 0.05 mg/ml. The ability of E. alatus to lower postprandial glucose was studied in streptozotocin-induced diabetic rats. A starch solution (1 g/kg) with and without E. alatus extract (500 mg/kg) was administered to diabetic rats by gastric intubation after an overnight fast. Plasma glucose levels were measured at 30, 60, 90, 120, 180, and 240 min. Plasma glucose levels were expressed in increments from baseline, and incremental areas under the response curve were calculated. Extract of E. alatus,which had an IC50 value of 0.272 mg/ml, inhibited yeast alpha-glucosidase activity in a concentration-dependent manner. A single oral dose of E. alatus extract significantly inhibited increases in blood glucose levels at 60 and 90 min (p<0.05) and significantly decreased incremental response areas under the glycemic response curve (p<0.05). These results suggest that E. alatus has an antihyperglycemic effect by inhibiting alpha-glucosidase activity in this animal model of diabetes mellitus.
MeSH Terms
Figure
Reference
-
1. Abrahaamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med. 2004. 164:486–491.2. Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997. 20:1822–1826.
Article3. Balflour JA, McTavish D. Acarbose. An update of its pharmacology and therapeutic use in diabetes mellitus. Drugs. 1993. 46:1025–1054.4. Baron AD. Postprandial hyperglycaemia and α-glucosidase inhibitors. Diabetes Res Clin Pract. 1998. 40:S51–S55.
Article5. Bastyr EJ, Stuart CA, Brodows RG, Schwartz S, Graf CJ, Zagar A, Robertson KE. IOEZ Study Group. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. Diabetes Care. 2000. 23:1236–1241.
Article6. Campbell RK, White JR, Nomura D. The clinical importance of postprandial hyperglycemia. Diabetes Educ. 2001. 27:624–637.
Article7. Centers for Disease Control and Prevention. Diabetes Surveillance Report. 1999. Atlanta, GA: US Department of Health and Human Services.8. Coniff RF, Shapiro JA, Robbins D, Kleinfield R, Seaton TB, Beisswenger P, McGill JB. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM. Diabetes Care. 1995. 18:817–824.
Article9. Haller H. The clinical importance of postprandial glucose. Diabetes Res Clin Pract. 1998. 40:S43–S49.
Article10. Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999. 22:960–964.
Article11. Inoue I, Takahashi K, Noji S, Awata T, Negishi K, Katayama S. Acarbose controls postprandial hyper-proinsulinemia in non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1997. 36:143–151.
Article12. Jenkins DJ, Wolever TM, Jenkins AL. Starchy foods and glycemic index. Diabetes Care. 1988. 11:149–159.
Article13. Jermendy G. Can type 2 diabetes mellitus be considered preventable? Diabetes Res Clin Pract. 2005. 68:S73–S81.
Article14. Joo HJ, Kang MJ, Seo TJ, Kim HA, Yoo SJ, Lee SK, Lim HJ, Byun BH, Kim JI. The hypoglycemic effect of Saururus chinensis Baill in animal models of diabetes mellitus. Food Science and Biotechnology. 2006. 15:413–417.15. Kim CH, Kim DI, Kwon CN, Kang SK, Jin UH, Suh SJ, Lee TK, Lee IS. Euonymus alatus (Thunb.) Sieb induces apoptosis via mitochondrial pathway as prooxidant in human uterine leiomyomal smooth muscle cells. Int J Gynecol Cancer. 2006. 16:843–848.
Article16. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.
Article17. Korea National Statistical Office. The cause of death Statistics 2005. Annual Report of on the Cause of Death Statistics. 2006. Seoul. Republic of Korea: Korea National Statistical Office.18. Lee H, Kim HK, Ha TY. Antitumor effect of winged Euonymus against chemically induced and malignant cell implanted-tumors in mice. Korean Journal of Immunology. 1993. 15:243–253.19. Li Y, Wen S, Kota BP, Peng G, Li GQ, Yamahara J, Roufogalis BD. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J Ethnopharmacol. 2005. 99:239–244.
Article20. Mooradian AD, Thurman JE. Drug therapy of postprandial hyperglycemia. Drugs. 1999. 57:19–29.21. Oh BY, Hwang SK, Cheong MY, Sin HS, Park BH, Lee JH, Kim S. Components and biological activity of aqueous extract isolated from winged stem of Euonymus alatus. Korean Journal of Food Science and Technology. 2005. 37:898–904.22. Park SH, Ko SK, Chung SH. Euonymus alatus prevents the hyperglycemia and hyperlipidemia induced by high-fat diet in ICR mice. J Ethnopharmacol. 2005. 102:326–335.
Article23. Saito N, Sakai H, Sekihara H, Yajima Y. Effect of an α-glucosidase inhibitor (voglibose), in combination with sulphonilureas, on glycaemic control in type 2 diabetes subjects. J Int Med Res. 1998. 26:219–232.
Article24. Sels JP, Huijberts MS, Wolffenbuttel BH. Miglitol, a new alpha-glucosidase inhibitor. Expert Opin Pharmacother. 1999. 1:149–156.25. Seo KS, Lim JK, Park JH, Kim CH, Chung GY, Jeong HJ. Antioxidative activity and biological properties in extracts of Euonymus alatus (Thnub.) Sieb. Korean Journal of Life Science. 2003. 13:1–8.26. Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, Min BH. Inhibitory effect of aqueous extract from the gall of Rhus Chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol. 2003. 85:283–287.
Article27. Soonthornpun S, Rattarasarn C, Leelawattana R, Setasuban W. Postprandial plasma glucose: a good index of glycemic control in type 2 diabetic patients having near-normal fasting glucose levels. Diabetes Res Clin Pract. 1999. 46:23–27.
Article28. Stand E, Baumgartl HJ, Fuchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999. 1:215–220.
Article29. The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in the diabetes control in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.30. UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998. 317:703–713.31. Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of -glucosidase inhibitors from Tochu-cha (Encommia ulmoides). Biosci Biotechnol Biochem. 1997. 61:177–178.
Article32. Youn JY, Park HY, Cho KH. Anti-hyperglycemic activity of Commelina communis L.: inhibition of α-glucosidase. Diabetes Res Clin Pract. 2004. 66S:S149–S155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory activity of Euonymus alatus against alpha-glucosidase in vitro and in vivo
- Effect of Euonymus alatus Extract on Antitumor Activity and Toxicity of Doxorubicin
- Protective effect of euonymus alatus extract on experimental liver injury in mice
- Dihydrobenzofuran Neolignans Isolated from Euonymus alatus Leaves and Twigs Attenuated Inflammatory Responses in the Activated RAW264.7 Macrophage Cells
- In Vitro α-Amylase, α-Glucosidase, Pancreatic Lipase, Xanthine Oxidase Inhibiting Activity of Agaricus bisporus Extracts