Korean J Pediatr Infect Dis.
2014 Apr;21(1):9-21.
Voriconazole Therapeutic Drug Monitoring is Necessary for Children with Invasive Fungal Infection
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children's Hospital, Seoul, Republic of Korea. pedeyc@gmail.com
- 2Department of Pediatrics, Seoul National University College of Medicine, Seoul, Republic of Korea.
- 3Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine, Seoul, Republic of Korea.
- 4Department of Pediatrics, Seoul National University Bundang Hospital, Gyeonggi-do, Republic of Korea.
- 5Cancer Research Institute, Seoul National University College of Medicine, Republic of Korea.
Abstract
- PURPOSE
To determine the clinical significance of voriconazole therapeutic drug monitoring (TDM) in the pediatric population.
METHODS
Twenty-eight patients with invasive fungal infections administered with voriconazole from July 2010 to June 2012 were investigated retrospectively. Fourteen received TDM, and 143 trough concentrations were analyzed. All 28 patients were assessed for adverse events and treatment response six weeks into treatment, and at the end.
RESULTS
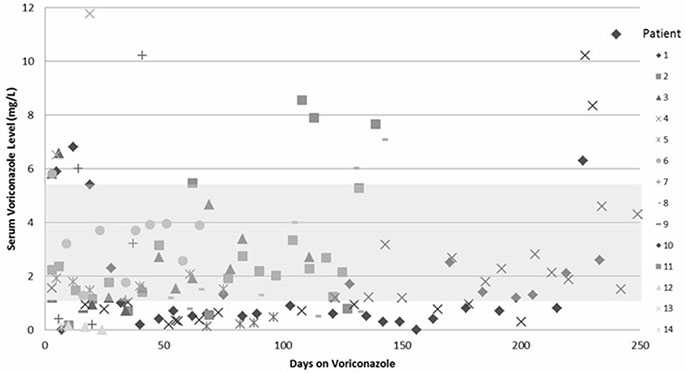
Out of 143 samples, 53.1% were within therapeutic range (1.0-5.5 mg/L). Patients administered with the same loading (6 mg/kg/dose) and maintenance (4 mg/kg/dose) dosages prior to initial TDM showed highly variable drug levels. Adverse events occurred in 9 of 14 patients (64.3%) in both the TDM and non-TDM group. In the TDM group, voriconazole-related encephalopathy (n=2, 14.3%) and aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation (n=8, 57.1%) occurred with serum levels in the toxic range (>5.5 mg/L), whereas blurred-vision (n=2, 14.3%) occurred within the therapeutic range (1.18 mg/L and 3.9 mg/L). The frequency of voriconazole discontinuation due to adverse events was lower in the TDM group (0.0% vs. 18.2%, P=0.481). Overall, 57.2% of the patients in the TDM group versus 14.3% in the non-TDM group showed clinical response after 6 weeks (P=0.055), whereas 21.4% in the TDM group versus 14.3% in the non-TDM group showed response at final outcome (P=0.664). In the TDM group, >67.0% of the serum levels were within therapeutic range for the first 6 weeks; however 45.5% were within therapeutic range for the entire duration.
CONCLUSION
Routine TDM is recommended for optimizing the therapeutic effects of voriconazole.
Keyword
MeSH Terms
Figure
Reference
-
1. Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002; 34:7–14.
Article2. Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin Infect Dis. 2006; 43:S3–S14.
Article3. Ashley ESD, Lewis R, Lewis JS, Martin C, Andes D. Pharmacology of systemic antifungal agents. Clin Infect Dis. 2006; 43:S28–S39.
Article4. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347:408–415.
Article5. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46:1813–1821.
Article6. Kauffman CA. The changing landscape of invasive fungal infections: epidemiology, diagnosis, and pharmacologic options. Clin Infect Dis. 2006; 43:S1–S2.
Article7. Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob Agents Chemother. 2002; 46:2546–2553.
Article8. Walsh TJ, Karlsson MO, Driscoll T, Arguedas AG, Adamson P, Saez-Llorens X, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother. 2004; 48:2166–2172.
Article9. Lat A, Thompson GR III. Update on the optimal use of voriconazole for invasive fungal infections. Infect Drug Resist. 2011; 4:43.10. Walsh TJ, Driscoll T, Milligan PA, Wood ND, Schlamm H, Groll AH, et al. Pharmacokinetics, safety, and tolerability of voriconazole in immunocompromised children. Antimicrob Agents Chemother. 2010; 54:4116–4123.
Article11. Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008; 47:674–683.
Article12. Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin Infect Dis. 2010; 50:27–36.
Article13. Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008; 46:201–211.
Article14. National Cancer Institute. 4.0. 2013. 03. 20. cited 2013 July 05. [Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm].15. Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook. 16th ed. Hudson, Ohio: Lexi-Comp, Inc.;2009. p. 1274–1276.16. Singh N. Treatment of opportunistic mycoses: how long is long enough? Lancet Infect Dis. 2003; 3:703–708.
Article17. Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012; 55:1080–1087.
Article18. Troke PF, Hockey HP, Hope WW. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob Agents Chemother. 2011; 55:4782–4788.
Article19. Ueda K, Nannya Y, Kumano K, Hangaishi A, Takahashi T, Imai Y, et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol. 2009; 89:592–599.
Article20. Choi SH, Lee SY, Hwang JY, Lee SH, Yoo KH, Sung KW, et al. Importance of voriconazole therapeutic drug monitoring in pediatric cancer patients with invasive aspergillosis. Pediatr Blood Cancer. 2013; 60:82–87.
Article21. Soler-Palacin P, Frick MA, Martin-Nalda A, Lanaspa M, Pou L, Rosello E, et al. Voriconazole drug monitoring in the management of invasive fungal infection in immunocompromised children: a prospective study. J Antimicrob Chemother. 2012; 67:700–706.
Article22. Michael C, Bierbach U, Frenzel K, Lange T, Basara N, Niederwieser D, et al. Voriconazole pharmacokinetics and safety in immunocompromised children compared to adult patients. Antimicrob Agents Chemother. 2010; 54:3225–3232.
Article23. Shimizu T, Ochiai H, Asell F, Shimizu H, Saitoh R, Hama Y, et al. Bioinformatics research on inter-racial difference in drug metabolism I. Analysis on frequencies of mutant alleles and poor metabolizers on CYP2D6 and CYP2C19. Drug Metab Pharmacokinet. 2003; 18:48–70.
Article24. Solis-Munoz P, Lopez JC, Bernal W, Willars C, Verma A, Heneghan MA, et al. Voriconazole hepatotoxicity in severe liver dysfunction. J Infect. 2013; 66:80–86.
Article25. Kim M, Youn H, Kim SB, Cho YH, Lee M, Cho S, et al. A case of visual and auditory hallucinations during intravenous voriconazole therapy. Infect Chemother. 2011; 43:421–424.
Article26. Imhof A, Schaer DJ, Schwarz U, Schanz U. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med Wkly. 2006; 136:739–742.27. den Hollander JG, van Arkel C, Rijnders BJ, Lugtenburg PJ, de Marie S, Levin MD. Incidence of voriconazole hepatotoxicity during intravenous and oral treatment for invasive fungal infections. J Antimicrob Chemother. 2006; 57:1248–1250.
Article28. Levin MD, den Hollander JG, van der Holt B, Rijnders BJ, Van Vliet M, Sonneveld P, et al. Hepatotoxicity of oral and intravenous voriconazole in relation to cytochrome P450 polymorphisms. J Antimicrob Chemother. 2007; 60:1104–1107.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Voriconazole Therapeutic Drug Monitoring is Necessary for Children with Invasive Fungal Infection
- A Case of Visual and Auditory Hallucinations during Intravenous Voriconazole Therapy
- Voriconazole-refractory invasive aspergillosis
- Management of Fungal Ocular Infection with Topical and Intracameral Voriconazole
- Invasive Fungal Infections in the Era of Antifungal Resistance