Ann Dermatol.
2011 Nov;23(4):424-431.
Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Chungnam National University, Daejeon, Korea. resina20@cnuh.co.kr
- 2Department of Anatomy, College of Medicine, Chungnam National University, Daejeon, Korea.
Abstract
- BACKGROUND
Autologous platelet-rich plasma has attracted attention in various medical fields recently, including orthopedic, plastic, and dental surgeries and dermatology for its wound healing ability. Further, it has been used clinically in mesotherapy for skin rejuvenation.
OBJECTIVE
In this study, the effects of activated platelet-rich plasma (aPRP) and activated platelet-poor plasma (aPPP) have been investigated on the remodelling of the extracellular matrix, a process that requires activation of dermal fibroblasts, which is essential for rejuvenation of aged skin.
METHODS
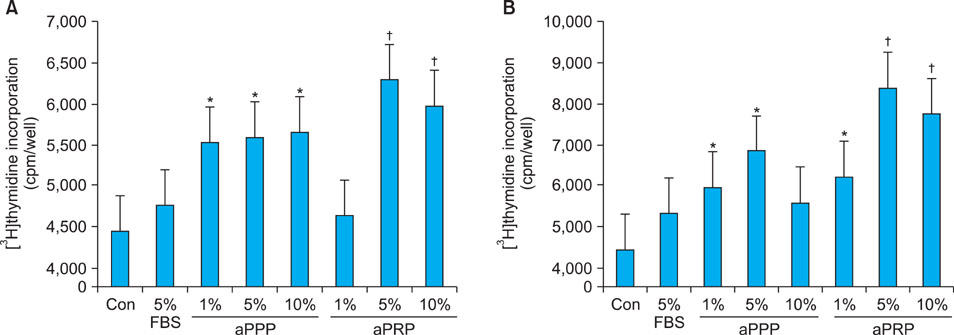
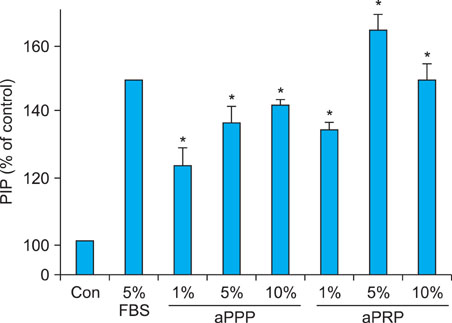
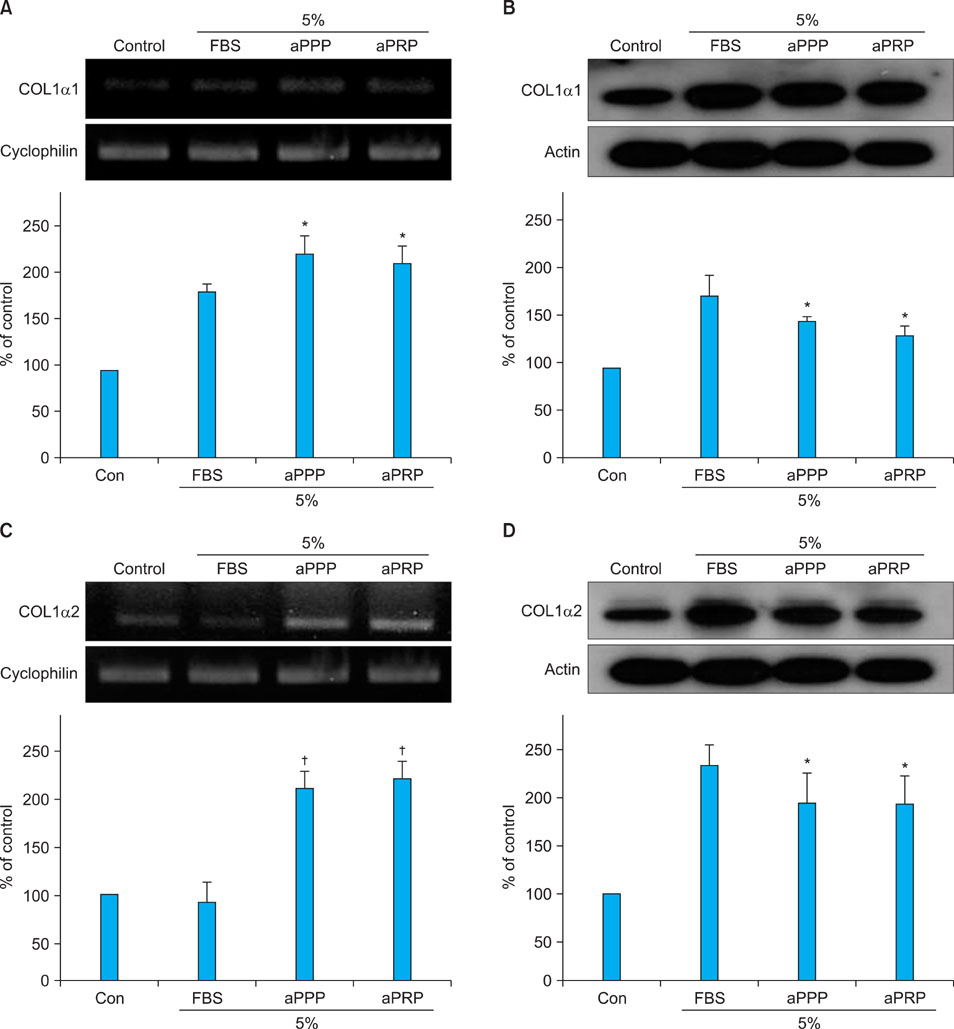
Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared using a double-spin method and then activated with thrombin and calcium chloride. The proliferative effects of aPRP and aPPP were measured by [3H]thymidine incorporation assay, and their effects on matrix protein synthesis were assessed by quantifying levels of procollagen type I carboxy-terminal peptide (PIP) by enzyme-linked immunosorbent assay (ELISA). The production of collagen and matrix metalloproteinases (MMP) was studied by Western blotting and reverse transcriptase-polymerase chain reaction.
RESULTS
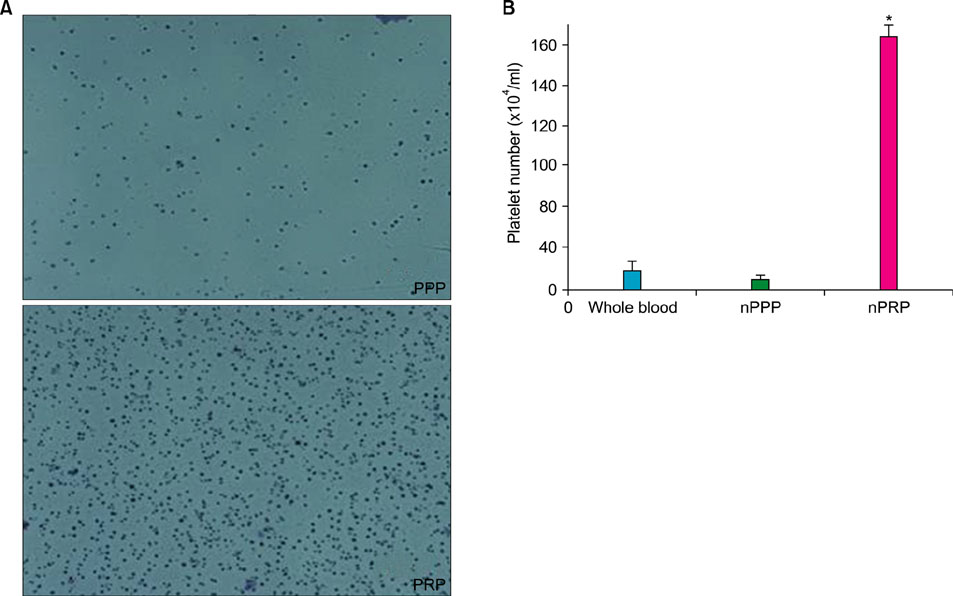
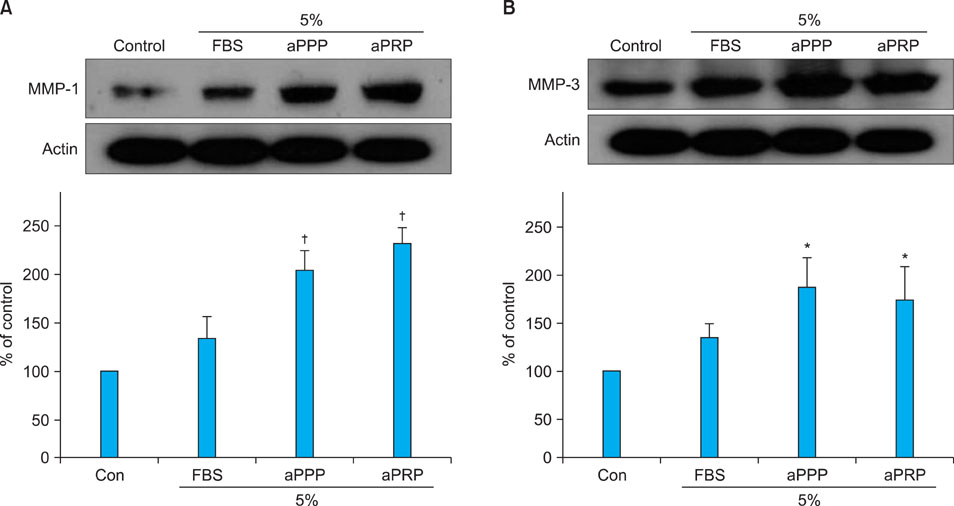
Platelet numbers in PRP increased to 9.4-fold over baseline values. aPRP and aPPP both stimulated cell proliferation, with peak proliferation occurring in cells grown in 5% aPRP. Levels of PIP were highest in cells grown in the presence of 5% aPRP. Additionally, aPRP and aPPP increased the expression of type I collagen, MMP-1 protein, and mRNA in human dermal fibroblasts.
CONCLUSION
aPRP and aPPP promote tissue remodelling in aged skin and may be used as adjuvant treatment to lasers for skin rejuvenation in cosmetic dermatology.
Keyword
MeSH Terms
-
Aged
Blood Platelets
Blotting, Western
Calcium Chloride
Cell Proliferation
Collagen
Collagen Type I
Dermatology
Enzyme-Linked Immunosorbent Assay
Extracellular Matrix
Fibroblasts
Humans
Matrix Metalloproteinases
Mesotherapy
Orthopedics
Plasma
Plastics
Platelet Count
Platelet-Rich Plasma
Rejuvenation
RNA, Messenger
Skin
Thrombin
Wound Healing
Calcium Chloride
Collagen
Collagen Type I
Matrix Metalloproteinases
Plastics
RNA, Messenger
Thrombin
Figure
Reference
-
1. Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of plateletrich plasma gel preparation. J Oral Maxillofac Surg. 2000. 58:297–300.
Article2. Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002. 17:184–190.3. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004. 62:489–496.
Article4. Freymiller EG. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004. 62:1046.
Article5. Wrotniak M, Bielecki T, Gaździk TS. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop Traumatol Rehabil. 2007. 9:227–238.6. Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2001. 24:483–488.
Article7. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:638–646.8. Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002. 18:27–33.
Article9. Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous plateletpoor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001. 107:229–237.
Article10. Yol S, Tekin A, Yilmaz H, Küçükkartallar T, Esen H, Caglayan O, et al. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008. 146:190–194.
Article11. Bernstein EF, Chen YQ, Kopp JB, Fisher L, Brown DB, Hahn PJ, et al. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J Am Acad Dermatol. 1996. 34:209–218.12. Petersen MJ, Hansen C, Craig S. Ultraviolet A irradiation stimulates collagenase production in cultured human fibroblasts. J Invest Dermatol. 1992. 99:440–444.
Article13. Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodam-aged adult human skin. J Invest Dermatol. 1995. 105:285–290.
Article14. Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J Cosmet Laser Ther. 2003. 5:25–34.
Article15. Cameron K. Review: chemotherapy plus supportive care improves survival and quality of life in advanced or metastatic gastrointestinal cancer more than supportive care alone. Evid Based Nurs. 2005. 8:18.
Article16. Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of plateletrich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008. 122:1352–1360.
Article17. Lee YS, Sohn KC, Jang S, Lee Y, Hwang C, Kim KH, et al. Anti-apoptotic role of S100A8 in X-ray irradiated keratinocytes. J Dermatol Sci. 2008. 51:11–18.
Article18. Le Pillouer-Prost A. Fibroblasts: whats new in cellular biology? J Cosmet Laser Ther. 2003. 5:232–238.
Article19. Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009. 53:96–102.
Article20. Gruber R, Karreth F, Frommlet F, Fischer MB, Watzek G. Platelets are mitogenic for periosteum-derived cells. J Orthop Res. 2003. 21:941–948.
Article21. Oprea WE, Karp JM, Hosseini MM, Davies JE. Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac Surg. 2003. 14:292–300.
Article22. Kocaoemer A, Kern S, Klüter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007. 25:1270–1278.
Article23. Kilian O, Flesch I, Wenisch S, Taborski B, Jork A, Schnettler R, et al. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res. 2004. 9:337–344.24. Soffer E, Ouhayoun JP, Dosquet C, Meunier A, Anagnostou F. Effects of platelet lysates on select bone cell functions. Clin Oral Implants Res. 2004. 15:581–588.
Article25. Gruber R, Karreth F, Frommlet F, Fischer MB, Watzek G. Platelets are mitogenic for periosteum-derived cells. J Orthop Res. 2003. 21:941–948.
Article26. Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH. Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg. 2005. 34:420–424.
Article27. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006. 17:212–219.
Article28. Karimipour DJ, Rittié L, Hammerberg C, Min VK, Voorhees JJ, Orringer JS, et al. Molecular analysis of aggressive microdermabrasion in photoaged skin. Arch Dermatol. 2009. 145:1114–1122.
Article29. Almeida Issa MC, Piñeiro-Maceira J, Farias RE, Pureza M, Raggio Luiz R, Manela-Azulay M. Immunohistochemical expression of matrix metalloproteinases in photodamaged skin by photodynamic therapy. Br J Dermatol. 2009. 161:647–653.
Article30. Redaelli A, Romano D, Marcianó A. Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. J Drugs Dermatol. 2010. 9:466–472.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Re: Histologic Evidence of New Collagen Formulation Using Platelet Rich Plasma in Skin Rejuvenation: A Prospective Controlled Clinical Study
- Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- Platelet-rich plasma, platelet derivatives, and their therapeutic importance in veterinary medicine
- Comparison of Intra-articular Hyaluronic Acid and Platelet-Rich Plasma Injection in Knee Osteoarthritis: Do the Results Differ in Geriatric Patients? A Retrospective Observational Study