Korean J Nutr.
2013 Apr;46(2):119-125. 10.4163/kjn.2013.46.2.119.
Effects of medicinal herb water extracts on expression of hepatic glucokinase, pyruvate dehydrogenase and acetyl-CoA carboxylase mRNA
- Affiliations
-
- 1Well-being Bioproducts RIC Center, Kangwon National University, Chuncheon 200-701, Korea. mchoe@kangwon.ac.kr
- 2Department of Bio-Health Technology, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 2269389
- DOI: http://doi.org/10.4163/kjn.2013.46.2.119
Abstract
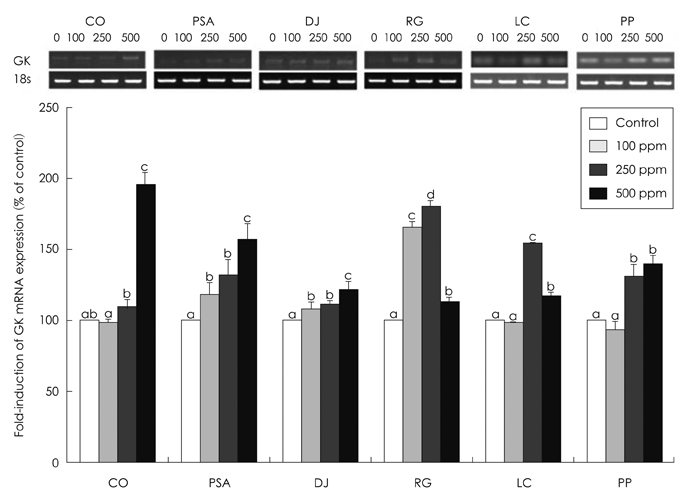
- We studied the anti-diabetic effects of medicinal herb water extracts on expression of hepatic glucokinase (GCK), pyruvate dehydrogenase (PDH), and acetyl-CoA carboxylase (ACC) mRNA. The medicinal herbs used for experiments were Cornus officinalis (CO), Paeonia suffruticosa Andrews (PSA), Discorea japonica Thunb. (DJ), Rehmannia glutinosa (RG), Lycium chinense (LC), and Pyrus pyrifolia (PP). For GCK mRNA expression, CO, RG, and LC water extracts exhibited a more effective activity than other extracts. Cells treated with RG and LC water extracts showed an increase in expression of PDH mRNA to 191% and 124%, respectively, compared to control. Expression of ACC mRNA was significantly higher in LC water extract. These data indicate that CO, RG, and LC water extracts stimulates expression of hepatic GCK, PDH, and ACC mRNA.
MeSH Terms
Figure
Cited by 2 articles
-
Exploration of optimum conditions for production of saccharogenic mixed grain beverages and assessment of anti-diabetic activity∗
Jae Sung Lee, Yun Hwan Kang, Kyoung Kon Kim, Yeong Kyeong Yun, Jun Gu Lim, Tae Woo Kim, Dae Jung Kim, Sang Yeon Won, Moo Hoan Bae, Han Seok Choi, Myeon Choe
J Nutr Health. 2014;47(1):12-22. doi: 10.4163/jnh.2014.47.1.12.Study of the mechanisms underlying increased glucose absorption in
Smilax china L. leaf extract-treated HepG2 cells∗
Yun Hwan Kang, Dae Jung Kim, Kyoung Kon Kim, Sung Mee Lee, Myeon Choe
J Nutr Health. 2014;47(3):167-175. doi: 10.4163/jnh.2014.47.1.167.
Reference
-
1. Egede LE, Ellis C. Diabetes and depression: global perspectives. Diabetes Res Clin Pract. 2010; 87(3):302–312.
Article2. Joo CN, Koo JH, Lee HB. Study on the hypoglycemic action of the fat soluble fraction of Panax ginseng C.A. meyer in streptozotocin induced diabetic rats. Korean J Ginseng Sci. 1993; 17(1):13–21.3. Joo CN, Kim SJ. Hypoglycemic action of the fat soluble fraction of Panax ginseng C.A. meyer in streptozotocin induced diabetic rats. Korean J Ginseng Sci. 1993; 17(2):101–108.4. Kim OK. Antidiabetic and antioxidative effect of Lycii fructus in streptozotocin-induced diabetic rats. Korean J Pharmacogn. 2009; 40(2):128–136.5. Park MJ, Kang SJ, Kim AJ. Hypoglycemic effect of Angelica gigas Naki extract in streptozotocin-induced diabetic rats. Korean J Food Nutr. 2009; 22(2):246–251.6. Takeda Y, Inoue H, Honjo K, Tanioka H, Daikuhara Y. Dietary response of various key enzymes related to glucose metabolism in normal and diabetic rat liver. Biochim Biophys Acta. 1967; 136(2):214–222.
Article7. Huang TH, Yang Q, Harada M, Uberai J, Radford J, Li GQ, Yamahara J, Roufogalis BD, Li Y. Salacia oblonga root improves cardiac lipid metabolism in Zucker diabetic fatty rats: modulation of cardiac PPAR-α-mediated transcription of fatty acid metabolic genes. Toxicol Appl Pharmacol. 2006; 210(1-2):78–85.
Article8. Kang SY, Paeng JR, Seo KS, Woo JT, Kim SW, Yang IM, Kim JW, Kim YS, Kim KW, Choi YK. Regulation of glucokinase gene expression and activity in the liver of diabetic rats. Korean J Med. 1994; 47(2):203–209.9. Lee EB, Choi BC, Cho TS. Pharmacological studies on ether fraction of Corni fructus. Yakhak Hoeji. 1985; 29(1):1–10.10. Kim OK. Antidiabetic and antioxidative effects of Corni fructus in streptozotocin-induced diabetic rats. J Korean Oil Chem Soc. 2005; 22(2):157–167.11. Joo HK, Jang DJ. Effects of Shanshuyu (Cornus officinalis Sieb) tea and market teas feeding on the hematology and liver function of rat. Korean J Diet Cult. 1989; 4(3):257–264.12. Seo KI, Lee SW, Yang KH. Antimicrobial and antioxidative activities of Corni fructus extracts. Korean J Postharvest Sci Technol. 1999; 6(1):99–103.13. Fukuhara Y, Yoshida D. Paeonol: a bio-antimutagen isolated from a crude drug, Moutan cortex. Agric Biol Chem. 1987; 51(5):1441–1442.
Article14. Mitsuo M, Maruyama H, Kameoka H. Essential oil constituents of "Moutan radicis cortex" Paeonia Moutan Sims. (P. suffruticosa Andrews). Agric Biol Chem. 1983; 47(12):2925–2927.15. You JK, Chung MJ, Kim DJ, Seo DJ, Park JH, Kim TW, Choe M. Antioxidant and tyrosinase inhibitory effects of Paeonia suffruticosa water extract. J Korean Soc Food Sci Nutr. 2009; 38(3):292–296.
Article16. Park S, Jun DW, Park CH, Jang JS, Park SK, Ko BS, Kim BJ, Choi SB. Hypoglycemic effects of crude extracts of Moutan radicis cortex. Korean J Food Sci Technol. 2004; 36(3):472–477.17. Lee ST, Chae YH. Botany of herbal resource. 1996. Seoul: Hakmun Publishing Co.;p. 130.18. Jeong HJ, Kim IH. Comparative studies on the antidiabetic activities of Rehmanniae radices -the effect of Rehmanniae radices extracts on streptozotocin-indeced hyperglycemia in rats-. Chung-Ang J Pharm Sci. 1990; 4:22–31.19. Cho YJ. Charactrization of biological activities of Rehmannia glutinosa extracts. J Life Sci. 2012; 22(7):943–949.
Article20. Cho SI. Effects of the Rehmanniae radix preparat on ovariectomized rats. Korean J Herbol. 2005; 20(4):61–67.21. Sheo HJ, Jun SJ, Lee MY. Effects of Lycii fructus extract on experimentally induced liver damage and alloxan diabetes in rabbits. J Korean Soc Food Nutr. 1986; 15(2):136–143.22. Kim BW, Roh KS. Study on the activity of GOT and GPT in the hepatotoxic rat treated Lycium chinense mill. Korean J Biomed Lab Sci. 2000; 6(3):187–192.23. Yoon CG, Kim HH, Chae SN, Oh MJ, Lee GH. Hepatic oxygen free radical and alcohol metabolizing enzyme activities in rats fed diets supplemented with Lycium chinense ethanol extract. J Korean Soc Food Sci Nutr. 2001; 30(4):668–672.24. Ahn BY, Gwak JS, Ryu SH, Moon GS, Choi DS, Park SH, Han JH. Protective effect of water extract of Lycii cordex radicis on lipid peroxidation of rat skin exposed to ultraviolet B radiation. Agric Chem Biotechnol. 2002; 45(4):218–222.25. Yu TJ. The food guide. 1989. Seoul: Munundang;p. 166.26. Choi HJ, Park JH, Han HS, Son JH, Son GM, Bae JH, Choi C. Effect of polyphenol compound from Korean pear (Pyrus pyrifolia Nakai) on lipid metabolism. J Korean Soc Food Sci Nutr. 2004; 33(2):299–304.27. An BJ, Lee JT, Kwak JH, Park JM, Lee JY, Son JH, Bae JH, Choi C. Biological activity of polyphenol group fraction from Korean pear peel. J Korean Soc Appl Biol Chem. 2004; 47(1):92–95.28. Chung MJ, Walker PA, Brown RW, Hogstrand C. ZINC-mediated gene expression offers protection against H2O2-induced cytotoxicity. Toxicol Appl Pharmacol. 2005; 205(3):225–236.
Article29. Nakamaru K, Matsumoto K, Taguchi T, Suefuji M, Murata Y, Igata M, Kawashima J, Kondo T, Motoshima H, Tsuruzoe K, Miyamura N, Toyonaga T, Araki E. AICAR, an activator of AMP-activated protein kinase, down-regulates the insulin receptor expression in HepG2 cells. Biochem Biophys Res Commun. 2005; 328(2):449–454.
Article30. Choi HJ, Kim SH, Oh HT, Chung MJ, Cui CB, Ham SS. Effects of Adenophora triphylla ethylacetate extract on mRNA levels of antioxidant enzymes in human HepG2 cells. J Korean Soc Food Sci Nutr. 2008; 37(10):1238–1243.
Article31. Ferre T, Pujol A, Riu E, Bosch F, Valera A. Correction of diabetic alterations by glucokinase. Proc Natl Acad Sci U S A. 1996; 93(14):7225–7230.
Article32. Muñoz MC, Barberà A, Domínguez J, Fernandez-Alvarez J, Gomis R, Guinovart JJ. Effects of tungstate, a new potential oral antidiabetic agent, in Zucker diabetic fatty rats. Diabetes. 2001; 50(1):131–138.
Article33. Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999; 274(1):305–315.
Article34. Pari L, Rajarajeswari N. Efficacy of coumarin on hepatic key enzymes of glucose metabolism in chemical induced type 2 diabetic rats. Chem Biol Interact. 2009; 181(3):292–296.
Article35. Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol. 2006; 38(7):1134–1145.
Article36. Kondeti VK, Badri KR, Maddirala DR, Thur SK, Fatima SS, Kasetti RB, Rao CA. Effect of Pterocarpus santalinus bark, on blood glucose, serum lipids, plasma insulin and hepatic carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2010; 48(5):1281–1287.
Article37. Ko BS, Kwon DY, Hong SM, Park S. In vitro anti-diabetic effects of crude extracts of Platycodi radix. Korean J Food Sci Technol. 2007; 39(6):701–707.38. Shimizu T, Parker JC, Najafi H, Matschinsky FM. Control of glucose metabolism in pancreatic β-cells by glucokinase, hexokinase, and phosphofructokinase. Model study with cell lines derived from β-cells. Diabetes. 1988; 37(11):1524–1530.
Article39. Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic β-cells and hepatocytes. Diabetes. 1990; 39(6):647–652.
Article40. Lee HA, Sim HS, Choi KJ, Lee HB. Hypoglycemic action of red ginseng components (II): investigation of the effect of fat soluble fraction from red ginseng on enzymes related to glucose metabolism in cultured rat hapatocytes. Korean J Ginseng Sci. 1998; 22(1):51–59.41. Kim HS, Ro YJ, Choe M. Effects of Cordyceps militaris on key enzymes of carbohydrate metabolism. J Korean Soc Food Sci Nutr. 2005; 34(10):1531–1535.
Article42. Choe M, Kim DJ, Lee HJ, You JK, Seo DJ, Lee JH, Chung MJ. A study on the glucose-regulating enzymes and antioxidant activities of water extracts from medicinal herbs. J Korean Soc Food Sci Nutr. 2008; 37(5):542–547.
Article43. Thampy GK, Haas MJ, Mooradian AD. Troglitazone stimulates acetyl-CoA carboxylase activity through a post-translational mechanism. Life Sci. 2000; 68(6):699–708.
Article44. Kim DJ, Chung MJ, You JK, Seo DJ, Kim JM, Choe M. Effect of medicinal plant water extracts on glucose-regulating enzyme activities in Goto-Kakizaki rat liver cytosol. J Korean Soc Food Sci Nutr. 2009; 38(10):1331–1335.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of medicinal herb water extracts on expression of hepatic glucokinase, pyruvate dehydrogenase and acetyl-CoA carboxylase mRNA
- Hypoglycemic effect of Rehmannie Radix Preparata (Sookjihwang) extract in streptozotocin-induced diabetic rats
- Regulation of acetyl CoA carboxylase mRNA in rat liver by high carbohydrate diet and insulin
- The Role of Pyruvate Dehydrogenase Kinase in Diabetes and Obesity
- The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet