Korean J Nutr.
2012 Oct;45(5):429-436. 10.4163/kjn.2012.45.5.429.
Characteristics of protein from red crab (Chionoecetes japonicus) shell by commercial proteases
- Affiliations
-
- 1Center of Smart Foods and Drugs and Food Science Institute, Inje University, Gimhae 621-749, Korea. fdsnsong@inje.ac.kr
- 2Clinical Research Institute CRL. Kyung Hee Universuty Hospital, Seoul 134-727, Korea.
- 3School of Culinary Art & Baking Technology, Dong-Ju College, Busan 604-080, Korea.
- 4Division of Marine Environment & Bioscience, Korea Maritime University, Busan 606-791, Korea.
- KMID: 2268737
- DOI: http://doi.org/10.4163/kjn.2012.45.5.429
Abstract
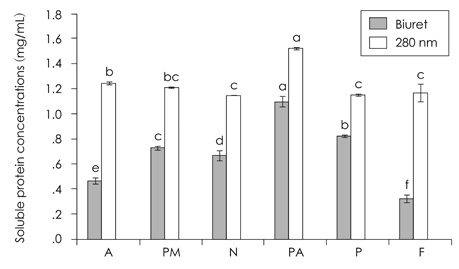
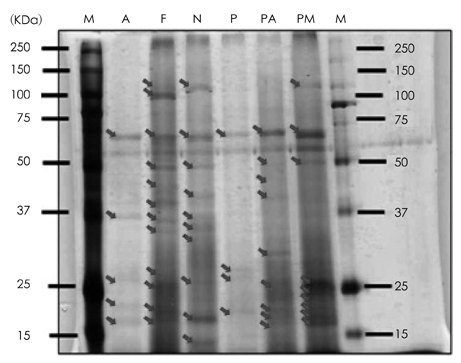
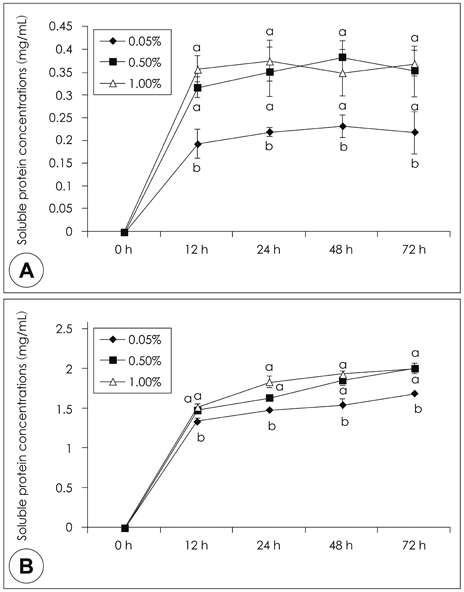
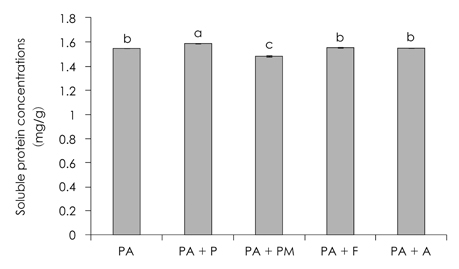
- This study was performed to examine the characteristics of protein of red crab (Chionoecetes japonicus) shell powder hydrolyzed by commercial proteases. Red crab shell was digested by commercial proteases, such as Protamex (P), Neutrase (N), Flavourzyme (F), Alcalase (A), Protease M (PM) and Protease A (PA). Protein yield analyzed by Biuret assay, absorbance at 280 nm and brix revealed that PA was the enzyme having the highest proteolytic activity. SDS PAGE showed that molecular weight of proteins produced by protease treatments was various and below 150 kDa. Combinational treatment of proteases (PA + P, PA + PM, PA + F, PA + A) was tried whether these increase protein hydrolysis from red crab shell powder compared to a PA single treatment. Soluble protein content was similar, but amino acid concentration by combinational treatments was higher than PA single treatment [PA + P 247.4 mg/g > PA + F (206.4 mg/g) > PA + A (133.4 mg/g) > PA + PM (59.1 mg/g) > PA (54.9 mg/g)]. Amino acid composition by combinational treatments was slightly different. Most abundant essential amino acids were phenylalanine, glycine, alanine, and leucine, whereas tyrosine and cystine were not detected.
MeSH Terms
-
Alanine
Amino Acids, Essential
Biuret
Cystine
Electrophoresis, Polyacrylamide Gel
Endopeptidases
Glycine
Hydrolysis
Leucine
Metalloendopeptidases
Molecular Weight
Peptide Hydrolases
Phenylalanine
Proteins
Subtilisins
Tyrosine
Alanine
Amino Acids, Essential
Biuret
Cystine
Endopeptidases
Glycine
Leucine
Metalloendopeptidases
Peptide Hydrolases
Phenylalanine
Proteins
Subtilisins
Tyrosine
Figure
Cited by 1 articles
-
Antioxidant activities of brown teff hydrolysates produced by protease treatment
Ye-Rang Yun, Sung-Hee Park
J Nutr Health. 2018;51(6):599-606. doi: 10.4163/jnh.2018.51.6.599.
Reference
-
1. Kim HS, Choi SG, Park CH, Han BW, Yang SK, Kang KT, Oh HS, Heu MS, Kim JS. Preparation and characteristics of surimi gel with red-tanner crab (Chionoecetes japonicus) paste. J Korean Soc Food Sci Nutr. 2005. 34(7):1103–1108.2. Seoung TJ, Choi SK, Byun GI. Studies on the processing of sauce by using red crab shell. Korean J Food Cult. 2008. 23(6):667–680.3. Jang JT, Seo WH, Baek HH. Enzymatic hydrolysis optimization of a snow crab processing by-product. Korean J Food Sci Technol. 2009. 41(6):622–627.4. Meyer SP, Chen HM, No HK, Lee KS. An integrated approach to recovery and utilization of Louisiana crawfish processing wastes. Making profits out of seafood wastes: Proceedings of the international conference on fish by-products. 1990. 1990 Apr 25-27; Anchorage, USA. Fairbanks: Alaska Sea Grant College Program, University of Alaska Fairbanks.5. Kim MJ, Nahmgung B, Kim BN, Lee SJ, Kim CJ, Cho YJ, Kim CT. Preparation and physicochemical characteristics of anchovy hydrolysates produced by high hydrostatic pressure and enzymatic hydrolysis treatment. Food Eng Prog. 2009. 13(2):85–91.6. Lee YC, Kim DS, Kim YD, Kim YM. Preparation of oyster (Crassostrea gigas) and sea mussel (Mytilus coruscus) hydrolyzates using commercial protease. Korean J Food Sci Technol. 1990. 22(3):234–240.7. Seoung TJ, Choi SK, Byun GI. Studies on the processing of sauce by using red crab shell. Korean J Food Cult. 2008. 23(6):667–680.8. Kim YJ, Shin TS, Oh HI. Solubility, emulsion capacity, and emulsion stability of protein recovered from red crab processing water. Korean J Food Nutr. 1996. 9(3):319–324.9. Kim YJ, Shin TS, Oh HI. Foaming capacity and foaming stability of protein recovered from red crab processing water. Korean J Food Nutr. 1996. 9(3):325–330.10. Park HK. In vitro antineoplastic effects of chitosan hydrolysates on various tumor cell lines. Korean J Food Nutr. 2009. 22(4):639–643.11. Yoon YC, An SI, Jeong AR, Han SE, Kim MH, Lee CK. Characteristics of whey protein (WPC-30) hydrolysate from cheese whey. J Anim Sci Technol. 2010. 52(5):435–440.
Article12. Jang SY, Sin KA, Park NY, Bang KW, Jeong YJ. Protein changes in soymilk and whole soymilk due to enzymatic hydrolysis. Korean J Food Preserv. 2008. 15(6):903–908.13. Jang SY, Gu YA, Park NY, Kim IS, Jeong YJ. Physicochemical property changes of whole soymilk dependent on hydrolysis conditions. Korean J Food Preserv. 2007. 14(4):394–399.14. Woo SH, Jhoo JW, Kim GY. Antioxidant activity of low molecular peptides derived from milk protein. J Korean Soc Food Sci Anim Resour. 2009. 29(5):633–639.
Article15. Lee WB. Study on the free radical scavenging activity of enzymatic extracts from velvet antler of elk (Cervus Canadensis) [MS dissertation]. 2007. Seoul: Konkuk University.16. Choi SK, Choi H, Lee JS. The characteristics of brown stock prepared by high pressure cooking. J East Asian Soc Diet Life. 2001. 11(4):281–288.17. Kang SI. Studies on the comparison of characteristics of fond de boeuf brun (beef brown stock) prepared by the traditional and the high pressure extraction methods [Ph. D. dissertation]. 2006. Gangneung: Kangnung National University.18. Bae GK, Byun GI, Choi SK. Quality characteristics of fish, crab and red-crab stock prepared by high pressure extract method. Korean J Culinary Res. 2007. 13(4):293–304.
Article19. Kim CW, Kim HS, Kim BY, Baik MY. Proteolysis of defatted rice bran using commercial proteases and characterization of its hydrolysates. Food Eng Prog. 2011. 15(1):41–47.20. Sargent MG. Fiftyfold amplification of the Lowry protein assay. Anal Biochem. 1987. 163(2):476–481.
Article21. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227(5259):680–685.
Article22. Kim CW, Park JW, Choi HJ, Han BK, Yoo SS, Kim BY, Baik MY, Kim YR. Hydrolysis of rice syrup meal using various commercial proteases. J Life Sci. 2011. 21(2):309–315.
Article23. Treimo J, Westereng B, Horn SJ, Forssell P, Robertson JA, Faulds CB, Waldron KW, Buchert J, Eijsink VG. Enzymatic solubilization of brewers' spent grain by combined action of carbohydrases and peptidases. J Agric Food Chem. 2009. 57(8):3316–3324.
Article24. Beak HH, Cadwallader KR. Enzymatic hydrolysis of crayfish processing by-products. J Food Sci. 1995. 60(5):929–935.
Article25. Adler-Nissen J. Enzymic hydrolysis of food proteins. 1986. New York: Elsevier Science Publishing Co;57–109.26. O'Meara GM, Munro PA. Selection of a proteolytic enzyme to solubilize lean beef tissue. Enzyme Microb Technol. 1984. 6(4):181–185.27. Kim JH, Yoo CJ, Sin KA, Jang SY, Park NY, Jeong YJ. Changes in properties of deer antler by proteolysis and extraction conditions. J Korean Soc Food Sci Nutr. 2011. 40(1):89–93.
Article28. Lee SH, Cho YJ, Kim S, Ahn BJ, Choi C. Optimal conditions for the enzymatic hydrolysis of isolated sesame meal protein. Agric Chem Biotechnol. 1995. 38(3):248–253.29. Gu YA, Jang SY, Park NY, Mun CR, Kin OM, Jeong YJ. Property changes of mung bean depending on hydrolysis conditions. Korean J Food Preserv. 2006. 13(5):563–568.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of protein from red crab (Chionoecetes japonicus) shell by commercial proteases
- Antiproliferatory Effects of Crab Shell Extract on Breast Cancer Cell Line (MCF7)
- Studies on the larval trematodes from brackish water crabs. Studies on a kind of Levinseniella
- Studies on Microphalloides japonicus
- A Study of Antifungal Activity with Rumex japonicus Houttuyn